Copy link
Adult Liver Transplantation: Anesthetic Considerations
Last updated: 10/16/2023
Key Points
- End-stage liver disease leads to portal hypertension (PH), which in turn, creates a unique pathophysiology that affects several organ systems in the body.
- Liver transplantation (LT) is the only proven treatment for end-stage liver disease.
- Anesthetizing a patient for LT requires an understanding of the complex pathophysiology and using both invasive and noninvasive monitoring to treat changes in hemodynamics, electrolyte abnormalities, and coagulation deficits that occur during the different stages of LT.
Introduction
- A cirrhotic liver is characterized by elevated intrahepatic resistance, which is termed intrahepatic PH. This decreases the blood flow through the hepatic sinusoids, and as a result, blood accumulates in the splanchnic circulation.
- The excess blood volume in the splanchnic circulation creates extrahepatic PH.
- Both intra- and extrahepatic PH will disrupt most organ systems in the patient.
- When the cirrhotic patient presents for LT, in addition to organ dysfunction due to PH, many organ systems will further be altered due to the high-volume resuscitation requirements that occur during LT.
- Successful care of the LT recipient requires the use of several monitoring techniques to manage the rapidly changing hemodynamic and hematologic disturbances.
- Please see the OA summary on surgical considerations for LT. Link
Patient Evaluation Prior to LT1-5
- In response to extrahepatic PH, the cirrhotic patient will have a vasodilated splanchnic vasculature to accommodate the excess splanchnic volume.
- The splanchnic vasodilation creates a low systemic vascular resistance (SVR).
- In order to generate adequate mean arterial pressures (MAP) to support organ perfusion, the cardiac output (CO) increases to overcome the low SVR.
- As a result, most cirrhotic LT patients will present with a hyperdynamic circulation:
- characterized by a low SVR and a compensatory high CO
- may be associated with a dynamic obstruction of the left ventricular outflow tract
- Despite a high CO, the low SVR often translates to a low MAP that may require vasopressor/inotropic support.
- If the CO is not appropriately compensating for the low SVR, the patient will need further cardiac workup before undergoing LT.
- Both intrahepatic and extrahepatic PH will affect most organ systems in the recipient, which often necessitates additional evaluation.
- Central nervous system: hepatic encephalopathy and cerebral edema
- Cardiac: cirrhotic cardiomyopathy and postcapillary pulmonary hypertension
- Pulmonary: hepatopulmonary syndrome and portopulmonary hypertension
- Renal: hepatorenal syndrome and total body volume overload with hyponatremia, ascites, and hepatic hydrothorax
- Hematologic: hypo- or hypercoagulopathy and disseminated intravascular coagulation (DIC)
- Knowing which complications are present allows the anesthesiologist to determine which monitors and drugs are required to safely anesthetize the patient.
Operating Room Set-up3
In addition to the standard American Society of Anesthesiologists monitors, the following monitors should be used.
Invasive Hemodynamic Monitoring
• Every LT recipient requires both an arterial line and a central line.
o The arterial line can be placed in the radial, brachial or femoral arteries.
o The central line should be placed above the diaphragm (internal jugular or subclavian vein) so it is not disrupted with caval crossclamping and/or torque on the cava.
• Use of a transesophageal echocardiogram (TEE) to monitor cardiac function in real time has become the standard of care. However, the presence of esophageal varices is a relative contraindication to TEE probe placement.
• For patients with portopulmonary hypertension, pulmonary artery catheters are essential for monitoring right heart hemodynamics intraoperatively, especially the pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR), at the time of reperfusion.
• Arterial waveform analysis for CO, stroke volume, stroke volume variation, and assessment of left heart function should be considered.
Noninvasive Hematologic Monitoring
• Frequent blood sampling is required to assess coagulopathy, electrolyte disorders and acid-base status. This is often achieved with point-of-care testing (POCT) performed in the operating room (OR).
• Focused interventions to manage rapidly changing coagulopathy require regular testing in the hospital laboratory, although some POCT such as thromboelastography (TEG) and rotational thromboelastometry can be performed quickly in the OR.
Other Equipment (Figure 1)
• Reliable large bore intravenous access is critical for the administration of fluids, blood products, vasopressors, and inotropes.
• Multiple infusion pumps to run vasopressor/inotrope infusions
• Method to deliver high volume of fluid rapidly (such as a Belmont or Level 1)
• Warming devices for both the patient and administered fluids
• Defibrillator pads and external cardiac pacemaker/defibrillator
• Arrangement for a perfusionist if the patient requires venovenous bypass (VVB) and a dialysis nurse if the patient requires intraoperative continuous renal replacement therapy.
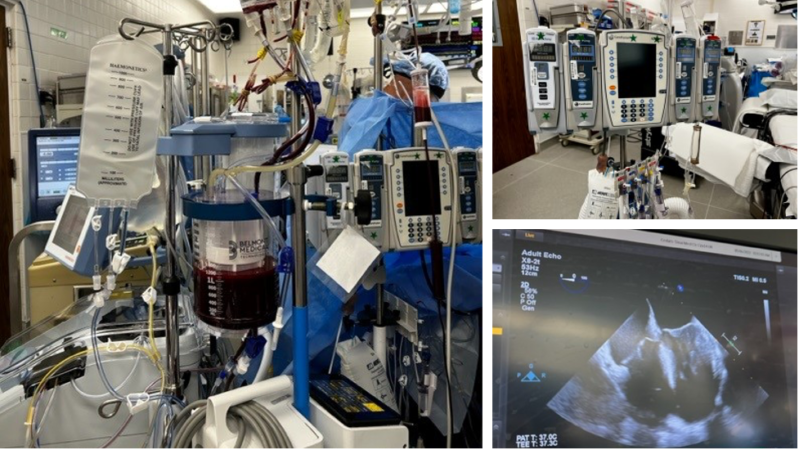
Figure 1. The OR setup for LT includes multiple infusion pumps, a system to deliver fluid rapidly (here seen with the Belmont® Rapid Infuser) and a TEE. Image courtesy: Jennifer Cutler, MD.
Abbreviations: OR, operating room; LT, liver transplantation; TEE, transesophageal echocardiogram
Induction of General Anesthesia
- The recipient should be considered full-stomach and the anesthesiologist should perform a rapid sequence induction with cricoid pressure.
- Induction agents should be titrated carefully to prevent a further decrease in the SVR.
- A hyperdynamic circulation often requires an arterial line to be placed preinduction to carefully watch the blood pressure with induction.
- Either inhalational or intravenous agents may be used to maintain general anesthesia.
- Prophylactic antibiotics should be administered before the surgical incision.
Intraoperative Hemodynamic Management1-4
Similar to the surgical considerations for LT which are divided into three stages (hepatectomy (or dissection), anhepatic, and reperfusion [or neohepatic]), the anesthetic management of the hemodynamic and hematologic alterations during LT also differ according to the stage of the surgery (see OA summary Adult Liver Transplantation: Surgical Considerations. Link).
Hepatectomy (Dissection) Stage
During this stage, the aim is to manage ongoing hemorrhage (Figure 2) and prepare the patient for the anhepatic stage by correcting coagulopathy and acid-base status, all while optimizing the circulating volume status.
Hemodynamic
- The cirrhotic patient is entering the LT with a hyperdynamic circulation that is dependent on a high preload to support their CO and MAP.
- In addition, manipulation of the diseased liver causes hemodynamic instability due to caval compression and/or torque of vascular structures.
- Intravascular volume replacement with a variety of fluids is necessary to maintain a high preload.
- Most volume is replaced with packed red blood cells (PRBCs) and fresh frozen plasma (FFP).
- If volume is required but blood products are not required, colloids are preferred over crystalloids.
- Vasopressors and inotropes, which will both raise the SVR as well as support cardiac contractility, are often required to supplement the volume replacement to support the MAP when the bleeding is profound.
Hematologic
- The cirrhotic patient is usually in a hypocoaguable state, manifested as a high international normalized ratio, low platelets, and low fibrinogen. Occasionally the cirrhotic patient will enter the LT in DIC.
- In addition, the cirrhotic patient is usually severely anemic.
- During hepatectomy, the aim for the anesthesiology team is to manage ongoing hemorrhage by correcting coagulopathy, acid-base status, and any electrolyte disturbances.
- Blood products can either come from the blood bank, or cell-salvage if the patient does not have underlying hepatocellular carcinoma (HCC).
- If the patient does have HCC, most centers will use cell-salvage after hepatectomy.
- The PRBCs and FFP are usually given in a 1:1 ratio to prevent dilutional coagulopathy.
- Additional calcium chloride (CaCl2) is often required to replace the ionized calcium that is bound by the blood bank preservative citrate.

Figure 2. Hemorrhage during the dissection phase. It is not uncommon for a patient to lose 2-5 times their circulating blood volume during dissection/hepatectomy. Image courtesy: Jennifer Cutler, MD.
Anhepatic Stage
During this stage, the aim is to hemodynamically support the patient while they are without a functioning liver and continue hematologic correction of the worsening acidosis and coagulopathy while anhepatic.
Hemodynamics
- The degree to which the anhepatic stage produces changes in the recipient’s hemodynamics is dependent on the surgical technique being used:
- In the classic LT (cLT) approach:
- The CO will decrease by 50% if the patient is not on VVB and by 30% if the patient is on VVB due to a decrease in the preload (see OA Summary Venovenous Bypass During Liver Transplantation. Link).
- At the same time there will be an increase in both the SVR (due to caval clamping) and heart rate (HR) (to compensate for the decrease in preload).
- If the patient has cirrhotic cardiomyopathy, they may not be able to increase the HR to compensate for the decrease in preload.
- In the piggyback LT approach, hemodynamic variables may produce a small decrease in preload or they may not fluctuate.
- In the classic LT (cLT) approach:
- Optimizing the circulating volume and using vasopressors are important to support the MAP during the ahepatic phase, especially if the surgeons are performing a cLT off VVB.
Hematologic
- Once anhepatic, coagulopathy and metabolic acidosis gradually worsen.
- Restricting blood return from the lower limbs and splanchnic bed during the anhepatic stage causes a build-up of lactate.
- Changes in ventilation and intravenous sodium bicarbonate are often required to treat the metabolic acidosis.
- Less blood products are given while anhepatic so as to prevent excess volume return to the heart with reperfusion.
- Steroids are given for immunosuppression per program guidelines.
- Serum potassium and ionized calcium levels must be optimized prior to reperfusion.
Reperfusion (Neohepatic) Stage
Restoration of blood flow through the allograft may cause severe cardiac instability due to the rapid bolus of cold, acidotic, and potentially hyperkalemic blood returning to the right heart and pulmonary arteries. The aim of the anesthesia team is to recognize and treat right heart dysfunction during reperfusion. A TEE to monitor cardiac function in real-time has become the standard of care.
Hemodynamics
- With reperfusion, the hemodynamics will again change:
- Preload increases as restoration of blood flow through the graft will result in a return of blood to the heart.
- SVR and ventricular contractility decrease, while PVR and PAP increase as the blood being returned is cold, acidotic and possibly hyperkalemic.
- Net result is an initial increase, followed by a decrease in the MAP with reperfusion.
- Vasopressors and inopessors are often required to overcome the decrease in both SVR and contractility with reperfusion.
- When the reperfused blood is severely cold and/or acidotic, or if excess blood volume is returned to the right heart, there can be an acute rise in the pulmonary pressures with resultant right heart dysfunction due to sudden dilation of the right ventricle.
- Lastly, hemodynamics can worsen if the patient develops reperfusion syndrome.4
- It is estimated to occur in 30% of LT cases and is associated with a prolonged cold ischemic time.
- It is characterized by a sustained decrease in MAP that is resistant to treatment with vasopressors and/or inotropes and a sustained increase in PAP.
- It is often associated with hemodynamically unstable dysrhythmias.
- It is often associated with recurrent fibrinolysis.
- In addition to receiving PRBC and FFP, the patient will now receive platelets and cryoprecipitate.
- If the patient was on VVB, the surgeons will have to first take the patient off VVB before platelets and cryoprecipitate are given.
- In the event of ongoing, nonsurgical bleeding, the patient may require antifibrinolytics or recombinant factors.
- After reperfusion, distinguishing bleeding due to surgical bleeding versus coagulopathy can be complex and often requires assessment with TEG monitoring.
- Improvements in acid-base status, coagulopathy and hemodynamic stability are good indicators of a return of hepatic function. Although, it is not uncommon for a patient to develop a metabolic alkalosis due to a new working liver metabolizing all the citrate that was present in blood bank PRBCs.
- Overtransfusion of blood products can lead to graft and splanchnic congestion and early allograft dysfunction (See OA Summary Allograft Function and Early Allograft Dysfunction Following Liver Transplantation Link)
Postoperative Considerations
- Most LT recipients go directly to the ICU following surgery for further hemodynamic and hematologic monitoring.
- Spontaneous ventilation and extubation at the conclusion of a LT is associated with an improvement in early graft blood flow.
- Pain control in extubated patients can be managed with patient-controlled analgesia pumps.
References
- Kashimutt S, et al. Anesthesia for liver transplantation. BJA Education. 2017; 17(1):35– 40. Link
- Adelmann D, Kronish K, Ramsay M. Anesthesia for liver transplantation. Anesthesiology Clinics. 2017; 35:491-508. PubMed
- Steadman RH. Anesthesia for liver transplant surgery. Anesthesiol Clin North Am. 2004; 22: 687 – 711. PubMed
- Wagener G. Liver transplantation: preanesthetic consultation and preparations. In: Post T (ed). UptoDate. 2023. Link
- Wagener G. Liver transplantation: anesthetic management. In: Post T (ed). UptoDate. 2023. Link
- Manning MW, Kumar PA, Maheshwari K, et al. Post-reperfusion syndrome in liver transplantation-An overview. J Cardiothorac Vasc Anesth. 2020; 34(2):501-11. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.