Copy link
Allograft Function and Early Allograft Dysfunction Following Liver Transplantation
Last updated: 10/23/2023
Key Points
- An allograft with normal function following liver transplantation (LT) will show evidence of new metabolic and synthetic activity along with decreasing serum hepatocellular and bile canaliculi enzyme levels.
- Early allograft dysfunction (EAD) identifies grafts with marginal function postoperatively.
- Patients who develop EAD following LT have a higher risk for allograft loss and mortality within the first 6 months following surgery.
- A potentially fatal subset of EAD is primary nonfunction (PNF), which represents loss of allograft function within the first 7 days.
- Attempts to decrease EAD and PNF include improved donor-recipient matching and machine-based perfusion technology.
Introduction
- Early recognition of patients with EAD is critical as organ support will be required while the allograft is not fully functional.
- Patients with PNF, however, have hepatocellular injury beyond the point of successfully responding to any support.
- It is hoped that improved donor-recipient compatibility and the use of machine-based perfusion technology will decrease the incidence of both EAD and PNF.
Allograft Function Following LT1
- A successfully transplanted organ will show early signs of allograft function.
- Intraoperatively, the allograft will
- begin to produce bile;
- start to metabolize citrate, evidenced by a metabolic alkalosis on an arterial blood gas result; and
- rapidly release hepatocellular transaminases into the blood as a response to the oxidative stress of the procurement process.
- Postoperatively, the allograft will
- continue to release transaminases whose levels usually peak on postoperative days 1 or 2 before decreasing;
- exhibit signs of improving metabolic and synthetic activity as evidenced by the return of the international normalized ratio (INR) to within normal limits, an increase in endogenous glucose production, and an increase in the serum platelet count;
- continue to produce and transport bile, although elevated bile canaliculi enzymes (alkaline phosphatase and GGT) return to normal levels slower than the transaminases; and
- buffer the circulating metabolic acidosis leading to a decrease in serum lactate levels.
Early Allograft Dysfunction
- EAD is a term that identifies those grafts with marginal function postoperatively.
- It can occur in allografts from both deceased and living donors.
- Patients with EAD often require cardiovascular and renal support.
- Even with support there remains a high risk of renal impairment, graft loss and patient mortality.
- Studies consistently show that the 1-, 3- and 5-year survival rates in those with EAD are all significantly lower than the survival rates in those who do not develop EAD.2
- Diagnosis of EAD
- There is a lack of consensus on the criteria that should be used to declare EAD.
- Many centers use the model proposed by Oltohoff et al. in 2010 which looks at AST/ALT levels, bilirubin levels, and the INR at or during the first 7 days after transplant.3 In this model, the increase in transaminase levels reflect hepatocellular injury while the increase in bilirubin and INR reflects compromised metabolic and synthetic function of the allograft.
- Agopian, et al. published a different risk assessment model where they considered EAD as a spectrum of disease.4 They devised the Liver Graft Assessment Following Transplantation (L-GrAFT) scoring tool, which looks at the trending of allograft function over the first 10 days posttransplant with higher scores reflecting a greater chance of graft failure in the first three months.
- Risk factors
- The incidence of EAD ranges from 23-46%.2 Not all patients who develop EAD need to be retransplanted, so it is important to be able to accurately identify those who do.
- The wide range in the incidence reflects the many potential risk factors for developing EAD. Factors which have been studied can be categorized as risks from the donor, risks from the recipient, intraoperative risk factors and postoperative risk factors (Table 1).
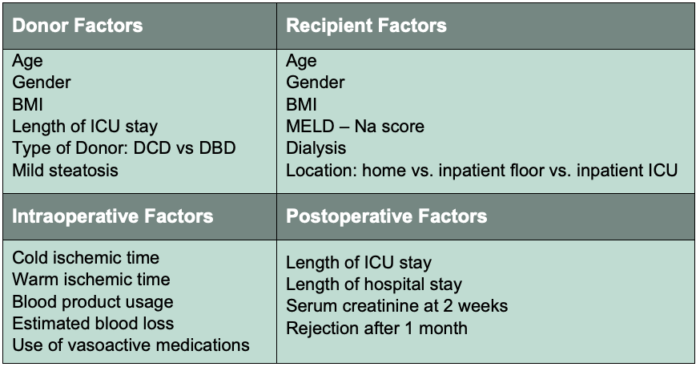
Table 1. Risk factors associated with the development of EAD.
Abbreviations: EAD, early allograft dysfunction; ICU, intensive care unit; DCD, donation after cardiac death; DBD, donation after brain death; BMI, body mass index; MELD-Na, model for end-stage liver disease-sodium. Adapted from Lee D, et al. Early allograft dysfunction after liver transplantation: an intermediate outcome measure for targeted improvements. Ann Hepatol. 2016; 15(1):53-60. PubMed.
- Each case of EAD is most likely due to a combination of factors but there is a small subset that appears to be consistently involved:
- Increased recipient MELD score
- Increased donor age
- Donation after cardiac death
- Increased blood product usage
- Increased allograft steatosis
- Increased cold ischemic time
- Increased operative time
- Pathophysiology of EAD
- EAD occurs as the result of a severe ischemia-reperfusion injury, which histologically shows acute inflammatory infiltration of the allograft. Sinusoidal endothelial cell damage with hepatocellular necrosis is a key factor.5
- The hepatocellular damage causes further inflammatory damage with a deterioration in both the synthetic and metabolic functions of the implanted organ.
Primary Nonfunction6
- PNF, previously known as early allograft loss, represents a subsection of EAD in which the allograft fails within the first 7 days after LT. It is estimated to occur in around 2% of all LT surgeries.6
- Unlike EAD, PNF will result in patient death unless retransplant is immediately available.
- There is no universal agreement on how to make the diagnosis of PNF, but a commonly used definition adopted across the US and UK requires that at least 2 of the following criteria must be present within the first 7 days after LT:
- AST > 10 000 IU/L
- INR > 3
- serum lactate >3 mmol/L
- the absence of bile production
Attempts to Decrease EAD and PNF
- As the need for donors has increased, transplant surgeons have increased the donor pool by accepting livers from known high-risk donors. These include those from donation-after-cardiac-death donors and organs with significant steatosis. Macrosteatosis up to 30% is often considered the threshold for safe transplantation, whereas grafts with moderate (30%-60%) or severe macrosteatosis (>60%) are considered too high a risk for the development of both EAD and PNF.6
- Attempts to decrease the incidence of EAD and PNF when using these donors have included the use of prognostic metrics to improve the match between donors and recipients, and the use of machine-based perfusion technology.
- Algorithms which attempt to better match donors and recipients do show that marginal donor allografts can safely be transplanted in lower MELD recipients.
- Machine-based perfusion technology allows the functional assessment of liver grafts by measuring bile production and lactate levels thereby allowing the transplant team to make a better risk assessment regarding the use of the donor liver. It also decreases the cold ischemic time. Both the functional assessment and the reduced cold ischemic time have greatly reduced the incidence of both EAD and PNF.
References
- Fedoravicius A and Charlton M. Abnormal liver tests after liver transplantation. Clin Liver Dis (Hoboken). 2016; 7(4):73-9. PubMed
- Lee D, Croome K, Shalev J, et al. Early allograft dysfunction after liver transplantation: an intermediate outcome measure for targeted improvements. Ann Hepatol. 2016; 15(1):53-60. PubMed
- Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010; 16:943–94. PubMed
- Agopian V, Harlander-Locke M, Markovic D, et al. Evaluation of early allograft function uning the liver graft assessment following transplantation risk score model. JAMA Surg. 2018; 153(5):436-44. PubMed
- Deschenes M. Early allograft dysfunction: Causes, recognition, and management. Liver Transpl. 2013; 19(S2):S6-S8. PubMed
- Hartog H, Hann A, Perera M. Primary nonfunction of the liver allograft. Transplantation. 2022; 106(1):117-28. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.