Copy link
Steroids in the ICU
Last updated: 06/12/2025
Key Points
- The hypothalamic-pituitary-adrenal (HPA) axis is a complex system that regulates hormone release, which is often disrupted in critically ill patients.
- Relative adrenal insufficiency is the most common type of adrenal insufficiency in critically ill patients.
- Laboratory data is frequently unreliable in critically ill patients, and most clinicians often treat adrenal insufficiency based on clinical judgment.
- There is a need to further investigate patient outcomes regarding steroid dosages in varying clinical presentations.
- Steroids, while useful in certain scenarios, can lead to unintended adverse effects further complicating care.
Introduction1,2
- Adrenal insufficiency is characterized by the inadequate secretion of cortisol, regardless of its etiology, and can be classified into three types: primary, secondary, and tertiary.
- Primary adrenal insufficiency is typically secondary to adrenal destruction, occurring when the adrenal cortex fails to produce adequate levels of cortisol despite appropriate adrenocorticotropic hormone (ACTH) levels.
- Secondary adrenal insufficiency occurs when cortisol levels are low due to inadequate secretion of ACTH.
- Tertiary adrenal insufficiency occurs when the cortisol-releasing hormone is dysregulated and is typically attributed to hypothalamic interference; this clinical presentation and management are similar to secondary adrenal insufficiency.
- Absolute adrenal insufficiency is rare in the intensive care unit, with a prevalence of less than 3%.
- Relative adrenal insufficiency, also known as critical illness-related corticosteroid insufficiency, is the most common type of adrenal insufficiency in critically ill patients and has been observed in up to 50% of septic patients.
- Factors such as prior steroid use, adrenal hemorrhage, medications (etomidate, ketoconazole), severe organ dysfunction, and inflammatory states are common within the intensive care unit and may contribute to adrenal insufficiency.
- Please see the OA summary on adrenal insufficiency and perioperative corticosteroids. Link
Pathophysiology3,4
- The adrenal cortex plays a crucial role in releasing steroids, including mineralocorticoids, glucocorticoids, and sex steroids.
- Mineralocorticoids assist in regulating salt and water balances through sodium and potassium transportation, and aldosterone is the primary mineralocorticoid.
- Cortisol is the primary glucocorticoid, playing a crucial role in regulating autonomic responses, metabolism, and immune and anti-inflammatory mechanisms.
- The HPA axis relies on negative feedback, in which increased cortisol levels can inhibit both corticotropin-releasing hormone and ACTH secretion, resulting in decreased glucocorticoid secretion and activity.
- Acute stressors, such as critical illness, trauma, and surgery, can dysregulate the HPA axis, leading to an increased release of cortisol and a decrease in cortisol breakdown. This can result in a blunted response to subsequent events.
- Corticosteroid-binding globulin levels are demonstrated to be decreased in patients with septic shock and are an independent predictor of mortality.
- Through various randomized clinical trials, hydrocortisone has been shown to lead to a pressor effect in septic shock and may offer a small mortality benefit in these patients.
Diagnosis2,3
- Adrenal insufficiency presents as malaise, weakness, fatigue, anorexia, weight loss, nausea, vomiting, abdominal pain, and/or diarrhea that alternates with constipation; additionally, hyponatremia and hyperkalemia are associated with mineralocorticoid deficiency as well as increased vasopressin levels.
- Adrenal crisis can be precipitated by acute adrenal insufficiency and may lead to refractory hemodynamic instability, a comatose state, and death.
- Hypoglycemia is frequently associated with adrenal insufficiency, especially in infants and patients with comorbidities such as diabetes, infection, chronic alcoholism, and critical illness.
- The ACTH stimulation test is the gold standard for diagnosis of adrenal sufficiency in which serum cortisol levels are measured at baseline and compared to 30 minutes and 60 minutes following administration of 250 mcg of cosyntropin (a synthetic form of ACTH); however, multiple studies demonstrate a poor response in critically ill patients given typically high levels of baseline cortisol with poor clearance and reduced responsiveness.4
- The ACTH stimulation test should not be the basis for initiating treatment, and most critical care providers will administer steroids utilizing clinical gestalt.
- Management for suspected adrenal insufficiency should not be delayed for laboratory testing; however, laboratory values such as electrolytes, aldosterone level, renin level, and morning cortisol may assist in guiding clinical decision-making.
Management5,6
- In acute adrenal crisis, clinicians must prioritize fluid resuscitation and electrolyte management while administering glucocorticoids such as an initial hydrocortisone 100 mg intravenous bolus followed by 50 mg every 6 hours with a taper to follow until hemodynamic stability improves, and patients with underlying chronic adrenal insufficiency may need lifelong steroid therapy.
- The 2021 Surviving Sepsis guidelines suggest initiating intravenous corticosteroids in refractory septic shock (when norepinephrine or epinephrine is ≤ 0.25 mcg/kg/min for four hours) as a weak recommendation (based on moderate-quality evidence), at a dose of 200 mg/day, administered as 50 mg intravenously every 6 hours or as a continuous infusion.6
- The Society of Critical Care Medicine suggests the following corticosteroid regimens based on the most common doses in available data:
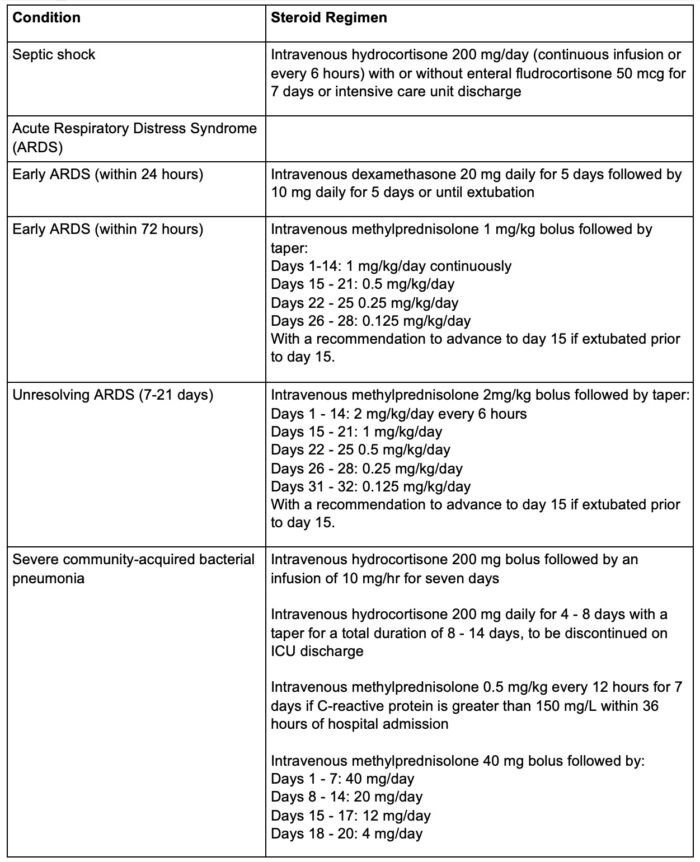
Table 1. Steroid dosing regimens. Adapted from Chaudhuri D, et al. Crit Care Med. 2024;52(5):e219-e233.5
- Furthermore, the Society of Critical Care Medicine also comments on the quality of such recommendations (Table 2).5
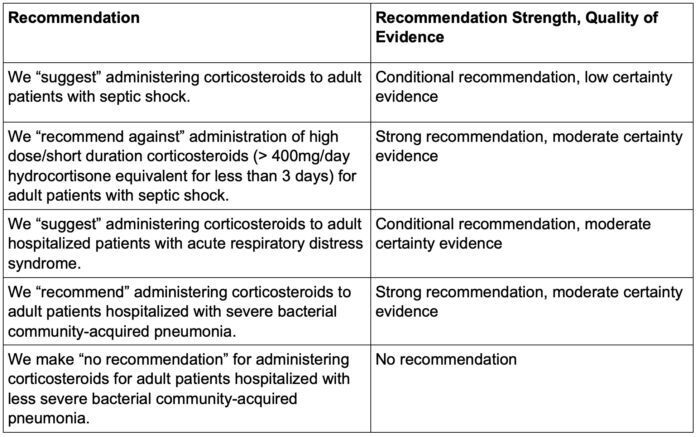
Table 2. Recommendations based on the quality of evidence. Adapted from Chaudhuri D, et al. Crit Care Med. 2024;52(5):e219-e233.5
- Multiple trials have demonstrated that corticosteroid use may reduce both short-term and long-term mortality in patients with sepsis or septic shock; however, subgroup analyses on corticosteroid type, duration of therapy, and dosage did not reveal any significant differences in these trials.
- While some studies demonstrate no long-term effects, corticosteroids are inexpensive and have the potential to assist with critical care-associated costs; however, there is a lack of data examining the cost-effectiveness of corticosteroids in patients with acute respiratory distress syndrome (ARDS).
- Glucocorticoids have been shown to be efficacious with or without the addition of a mineralocorticoid agent; however, there is a theoretical benefit of adding mineralocorticoid therapy, such as fludrocortisone, in severe septic shock. Further studies are needed to investigate a previous retrospective claim of mortality benefit.7
- The duration of steroid use remains under debate; however, steroids are tapered off based on clinical improvement to avoid lasting adverse effects.
- At higher dosages, hydrocortisone exhibits weak mineralocorticoid properties; in contrast, some steroids, such as dexamethasone, lack any mineralocorticoid properties.8
- Steroids vary in potency and duration of action. While conversion calculators exist, there is a dearth of data investigating the effects of prednisone and dexamethasone in various critical clinical scenarios.
Additional Indications
- Steroid therapy is obviously not limited to critical illnesses, and varying dosages as well as choice of glucocorticoid have been routinely utilized for treatment of croup, improvement of mortality in pneumocystis carinii prophylaxis, correction of hyperthyroidism, prevention of hearing loss in Hemophilus influenzae meningitis, improvement in pain scores for patients with cancer, interstitial lung disease, and autoimmune conditions.8
- Respiratory conditions such as ARDS, COVID-19 pneumonia, and community-acquired pneumonia may also benefit from steroid therapy.5
- Steroid use in influenza has been associated with higher mortality, no effect on mechanical ventilation-free days, longer ICU length of stay, and increased risk of secondary infection.9
Adverse Effects
- Whether intentional or unintentional, steroid therapy affects multiple organ systems and can result in several adverse effects.8
- Fluid and electrolyte disturbances (hypernatremia, increased potassium and calcium excretion)
- Gastrointestinal problems (nausea, vomiting, weight loss/gain, peptic ulcer)
- Endocrinological dysregulation (secondary adrenal insufficiency, Cushingoid state, hyperglycemia, insulin resistance, menstrual irregularities)
- Thromboembolism
- Hypertension
- Heart failure exacerbation
- Infections
- Avascular necrosis
- Mood changes
- Poor wound healing
- Muscle weakness
- While it is debated whether corticosteroids contribute significantly to gastrointestinal bleeding, secondary infections, neuromuscular weakness, and neuropsychiatric events, precautions are typically taken by providing gastrointestinal prophylaxis, having a high suspicion for concurrent infection, promoting early mobilization, and enforcing delirium precautions within the intensive care unit.10
- Corticosteroids increase the risk of hyperglycemia (high certainty) and probably increase the risk of hypernatremia (moderate certainty).10
- Corticosteroid utilization in patients with surgical sepsis without shock was underrepresented in clinical trials and requires further investigation as there exists theoretical harm with wound healing and anastomoses.5
References
- Malerba G, Romano-Girard F, Cravoisy A, et al. Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation. Intensive Care Med. 2005;31(3):388-92. PubMed
- Lamberts SW, Bruining HA, de Jong FH. Corticosteroid therapy in severe illness. N Engl J Med. 1997;337(18):1285-92. PubMed
- Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing's syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2014;4(2):739-69. PubMed
- Boonen E, Vervenne H, Meersseman P, et al. Reduced cortisol metabolism during critical illness. N Engl J Med. 2013;368(16):1477-88. PubMed
- Chaudhuri D, Nei AM, Rochwerg B, et al. 2024 focused update: Guidelines on use of corticosteroids in sepsis, acute respiratory distress syndrome, and community-acquired pneumonia. Crit Care Med. 2024;52(5):e219-e233. PubMed
- Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181-1247. PubMed
- Matthay MA, Dahabreh IJ, Thompson BT. Should We Add Fludrocortisone to Hydrocortisone for Treatment of Septic Shock? JAMA Intern Med. 2023;183(5):460-461. PubMed
- Zoorob RJ, Cender D. A different look at corticosteroids. Am Fam Physician.1998;58(2):443-50. PubMed
- Martin-Loeches I, Torres A. Corticosteroids for CAP, influenza and COVID-19: when, how and benefits or harm? Eur Respir Rev. 2021;30(159). PubMed
- Chaudhuri D, Israelian L, Putowski Z, et al. Adverse effects related to corticosteroid use in sepsis, acute respiratory distress syndrome, and community-acquired pneumonia: A systematic review and meta-analysis. Crit Care Explor. 2024;6(4):e1071. PubMed
Other References
- Wright M, Gravenstein N. Adrenal insufficiency and perioperative corticosteroids. OA summary. Created May 24, 2024. Accessed June 12, 2025. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.