Copy link
Fontan Circulation
Last updated: 06/04/2025
Key Points
- The Fontan procedure is the final of three palliative surgeries performed for patients born with single-ventricle anatomy.
- The surgery reroutes systemic venous blood flow directly to the pulmonary arteries, bypassing the subpulmonic right ventricle.
- This creates a nonpulsatile pulmonary circulation that relies on blood flow generated by the kinetic energy produced by the pulsatile single left or right systemic ventricle.
- After an initial period of excellent physiological health, Fontan patients gradually experience multisystem complications due to chronically elevated venous pressures.
- Anesthetic management in patients with Fontan circulation requires unique hemodynamic and ventilatory considerations, as well as an understanding of the multisystemic complications associated with this condition.
Introduction
- The Fontan procedure is part of a staged palliative surgical strategy for congenital heart defects with a single functional ventricle, including hypoplastic left and right heart syndrome, tricuspid atresia, and unbalanced atrioventricular canal defects.1,2
- The procedure reroutes systemic venous return directly to the pulmonary arteries, bypassing return of blood to the right atrium and the single ventricle and thereby eliminating the need for a subpulmonary pump.
- The resulting Fontan circulation creates nonpulsatile pulmonary blood flow, which is responsible for the clinical implications and multisystem complications observed in these patients.2-4
Surgical Stages
- Described by Dr. François Fontan in 1971 for the surgical management of tricuspid atresia, the Fontan procedure represents the final stage of single-ventricle palliation.
- The staged surgical approach promotes pulmonary vascular development and gradually reduces volume load on the single ventricle.1
- A fenestration is often created between the Fontan baffle and the common atrium in order to serve as a “pop-off” during times of elevated Fontan pressures. This allows blood to bypass the lungs, preserving cardiac output during periods of elevated pulmonary resistance at the cost of a lowered oxygen saturation (Figure 2).2
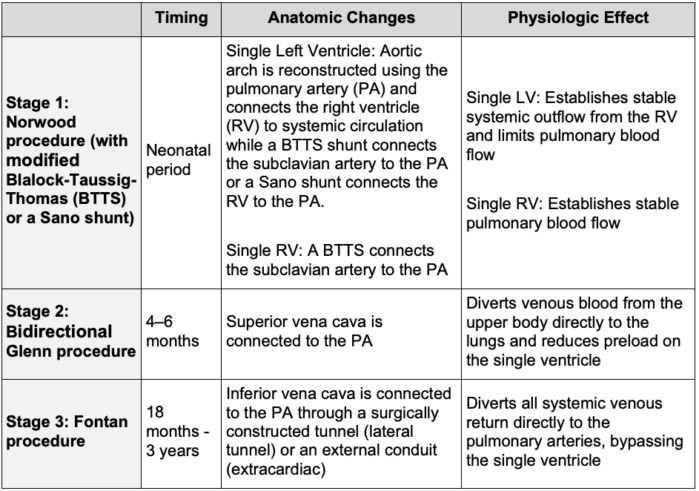
Table 1. Surgical stages of Fontan circulation1,3
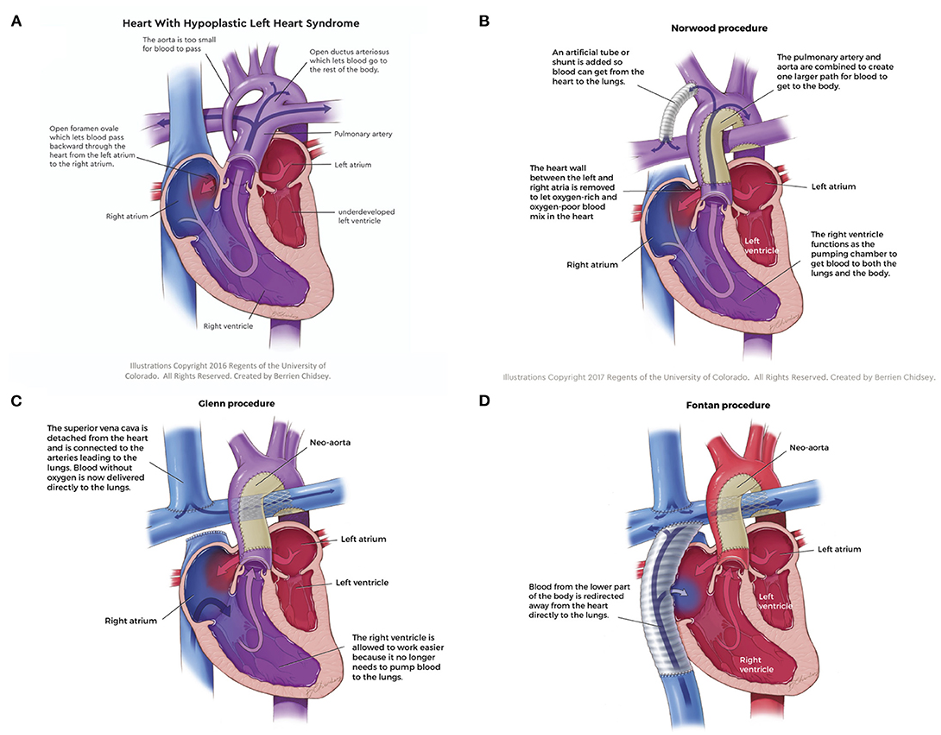
Figure 1. Anatomy of hypoplastic left heart syndrome and the surgical stages culminating in the Fontan circulation; (A) Hypoplastic left heart syndrome, (B) Norwood procedure, (C) Glenn procedure, (D) Fontan procedure. Source: Ferrari MR, Di Maria MV, Jacot JG. Review on mechanical support and cell-based therapies for the prevention and recovery of the failed Fontan-Kreutzer circulation. Front Pediatr. 2021;8:627660. CC BY 4.0. Link
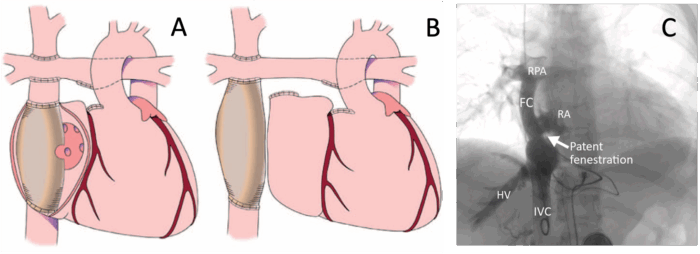
Figure 2. Fontan Fenestration. (A) Fenestrated lateral tunnel (intra-atrial) Fontan. (B) Unfenestrated extracardiac Fontan. (C) Angiography of an extracardiac Fontan with a patent fenestration. FC, Fontan conduit; HV, hepatic vein; IVC, inferior vena cava; RA, right atrium; RPA, right pulmonary artery. A and B reproduced with permission from Jolley M, et al Anesth Analg. 2015;121(1):172-82. Link. C from Wittczak A, et al. Clin Case Rep. 2023;11(5):e7222. CC BY-NC 4. Link
Fontan Physiology
- In the Fontan circulation, a single ventricle drives flow across three resistance beds (systemic, pulmonary, and total cavopulmonary) arranged in series (Figure 3A).4
- Because there is no subpulmonary ventricle, Fontan patients require 75% more hydraulic power per unit of forward flow, despite having a cardiac index approximately 37% lower than that of individuals with normal cardiac anatomy.4
- Ventricular preload is gradient-driven from the venous reservoir across the pulmonary vascular resistance (PVR) (Figure 3B). Preload can be increased by either increasing venous volume or decreasing venous capacitance; however, increases in venous pressure simultaneously elevate both preload and afterload on the single ventricle.4
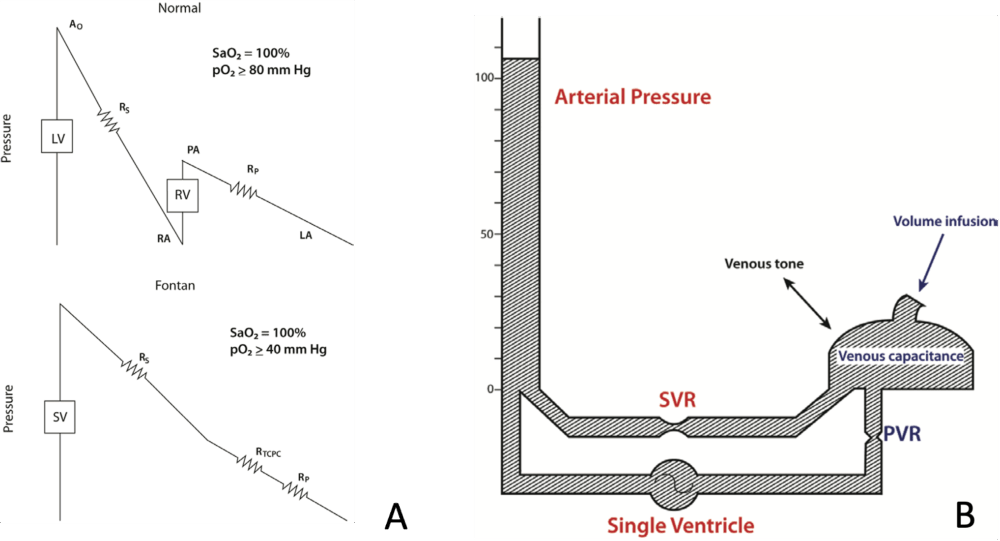
Figure 3. A. Schematic of normal and Fontan Circulations. B. Hydraulic schematic of Fontan circulation. In a Fontan circulation, the single ventricle pumps blood through systemic and pulmonary resistances arranged in series. Ao, aorta; LA, left atrium; LV, left ventricle; PVR, pulmonary vascular resistance; RA, right atrium; RP, pulmonary resistance; RS, systemic resistance; RTPVC, total cavopulmonary resistance; RV, right ventricle; SV, single ventricle; SVRr, systemic vascular resistance. From: Jolley M, et al. Anesth Analg. 2015;121(1):172-82.
- The Fontan circulation is more dependent than a biventricular circulation on negative intrathoracic pressure from respiration and skeletal muscle contraction to maintain venous return to the systemic ventricle and sustain cardiac output. Approximately 30% of systemic venous flow to the pulmonary arteries is dependent on inspiration in the Fontan circulation, compared with 15% in normal two-ventricle patients.1,3,5
- Pulmonary blood flow is highly sensitive to changes in PVR and central venous pressure (CVP). Small increases in PVR can significantly reduce pulmonary blood flow, leading to decreased preload and cardiac output. Stressors such as hypoxia, hypercarbia, acidosis, or high peak airway pressures can disrupt this balance, and impair oxygen delivery and cardiac output.1-3,5
- A chronic nonpulsatile pulmonary circulation results in progressive increases in PVR up to four times normal. This results from endothelial dysfunction caused by reduced nitric oxide bioavailability and impaired pulmonary capillary recruitment.3-5
- Cardiac arrhythmias are common and impair the ability to augment cardiac output by limiting heart rate responsiveness and the atrial contribution to ventricular filling.3
- Chronic cyanosis is typical even in well-functioning circuits, with oxygen saturations frequently remaining below 92% and rarely more than 95%.1
Long-Term Survival and Fontan Failure
- Fontan survival has improved significantly with modern surgical techniques, with perioperative mortality below 2% and transplant-free survival reaching 90% at 10 years, 83% at 20 years, and 70% at 25 years.2
- The most common causes of death in Fontan patients are heart failure, thromboembolism, and sudden cardiac death from arrhythmias.2
- The chronic elevation in CVP is central to Fontan failure, impairing venous return and causing congestion in both the systemic and pulmonary circulations.2-4
- Fontan failure is a multifactorial syndrome rather than a single diagnosis, often developing gradually with a variety of clinical presentations that differ depending on the patient’s specific single-ventricle anatomy.4
Complications of Fontan Circulation
- Chronic elevation in systemic venous pressure impairs cavopulmonary blood flow and underlies many of the pathophysiologic mechanisms implicated in Fontan failure.2-4
- Fontan-associated liver disease (FALD) develops universally due to chronic hepatic congestion caused by a chronically elevated CVP and a lack of valves in the hepatic veins. Elevated CVP results in progressive liver dysfunction including fibrosis, cirrhosis, portal hypertension, ascites, and, in rare cases, hepatocellular carcinoma.2-4
- Atrioventricular (AV) valve regurgitation is common in Fontan patients, with failure rates as high as 56% for common AV valves at 25 years and is associated with more than double the risk of Fontan cardiac failure.1
- Arrhythmias affect 58% of Fontan patients at 20 years and 76% at 30 years, with sinus node dysfunction and atrial tachyarrhythmias being the most common. These arrhythmias increase the risk of thromboembolism and tachycardia-induced cardiomyopathy.1
- Thromboembolic risk in Fontan patients can be as high as 20%, influenced by clotting abnormalities including deficiencies in protein C, protein S, antithrombin III, and increased platelet reactivity. This risk is further increased by nonpulsatile pulmonary blood flow, potential arrhythmias, and the presence of prosthetic grafts.3
- Coagulopathy is also a hallmark of progressive FALD, reflecting impaired hepatic synthesis of clotting factors, thrombocytopenia, and a paradoxical predisposition to both bleeding and thrombosis.4
- Restrictive lung disease affects approximately 89% of Fontan patients, resulting from pulmonary hypoplasia and impaired chest wall mechanics.2,3
- Lymphatic complications are common in Fontan patients due to lymphatic congestion caused by elevated venous pressures, low cardiac output, endothelial dysfunction, and chronic inflammation.2-4
- Protein-losing enteropathy results from intestinal lymphatic congestion and is characterized by hypoalbuminemia, ascites, and peripheral edema.2-4
- Plastic bronchitis is a rare but life-threatening complication that results from lymphatic leakage into the bronchial tree. This leads to the formation of obstructive bronchial casts that cause significant respiratory distress and often requires management with urgent bronchoscopy and cast removal.1,4
- Additional long-term complications of Fontan circulation are summarized in Table 2.
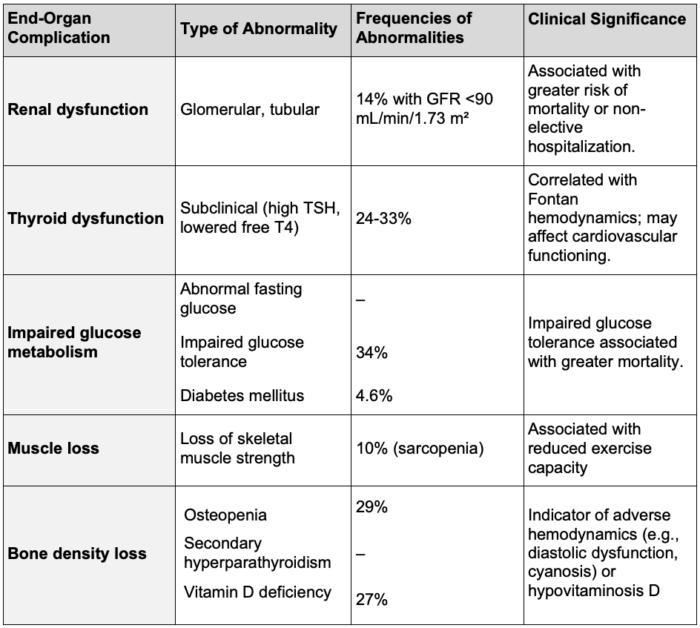
Table 2. End-organ dysfunction in Fontan patients. Adapted from: AlZahrani A, Rathod R, Krimly A, Salam Y, AlMarzoog AT, Veldtman GR. The Adult Patient with a Fontan. Cardiol Clin. 2020;38(3):379-401.
Anesthetic Considerations
- Comprehensive preoperative assessments are essential for Fontan patients who have a significantly increased perioperative risk, with complication rates reported up to 31%.2
- Inadequate preoperative evaluation accounts for up to 40% of adverse events in adults with congenital heart disease undergoing noncardiac surgery.2
- A detailed review of prior surgeries, cardiac and hepatic function, and the presence of right-to-left shunts (fenestration, baffle leak, and/or venovenous collaterals) is essential to guide anesthetic planning.2,4
- Preoperative assessment of cardiac status in Fontan patients is challenging due to their complex anatomy and multifactorial heart failure physiology. Evaluation with exercise testing, echocardiography, and cardiac magnetic resonance imaging or computed tomography should be considered.2
- Optimizing perioperative volume status is crucial due to the preload dependence of Fontan circulation. Strategies include minimizing fasting, careful fluid titrations, multiple sites of IV access, and potential use of central venous catheters.2
- Patient positioning can also significantly affect hemodynamics, with the potential for head-down and lateral positions to increase pulmonary vascular resistance and head-up and prone positions to reduce venous return.2
- Arterial lines are generally recommended for continuous blood pressure monitoring and blood gas analysis.2,4
- Vasopressor and inotropic support should be adapted to Fontan physiology, with vasopressin having a theoretical advantage of avoiding pulmonary vasoconstriction, and milrinone commonly used as an inotrope.2
- Ventilatory management requires minimizing positive intrathoracic pressures to preserve pulmonary blood flow and cardiac output. Recommended techniques include using low positive end-expiratory pressure, low mean airway pressures, short inspiratory time, and prolonged expiratory time. Low respiratory rates and tidal volumes of 8-10 mL/kg help maintain normocarbia and minimize PVR.2,4
- Spontaneous ventilation increases systemic venous return by creating negative intrathoracic pressure and should be prioritized when feasible.2,4
- Pulmonary vasodilators, such as nitric oxide, can reduce PVR by limiting hypoxemic pulmonary vasoconstriction.
- Regional anesthesia avoids positive pressure ventilation, facilitates early ambulation, and reduces the need for respiratory-depressant narcotics.2
- Neuraxial techniques require caution due to increased bleeding risk from high venous pressures and synthetic liver dysfunction.2,4
- Laparoscopic surgery in Fontan patients impairs venous return and increases PVR. It is recommended to use low insufflation pressures (less than 10 mmHg), invasive monitoring, and maintain a low threshold to convert to open surgery.2
- Pregnancy further increases thromboembolic risk and can cause cardiovascular collapse though aortocaval compression. Vaginal delivery is preferred due to fewer embolic events and can be assisted with epidural analgesia to shorten the second stage and reduce Valsalva-induced decreases in venous return. If general anesthesia is needed, ketamine or etomidate are preferred for their hemodynamic stability.2
- The postoperative period accounts for up to 50% of complications in Fontan patients. Management should include close monitoring of volume status, early reinstitution of enteral intake and pulmonary vasodilators, and multimodal pain control.2,4
References
- AlZahrani A, Rathod R, Krimly A, et al. The adult patient with a Fontan. Cardiol Clin. 2020;38(3):379-401. PubMed
- McNamara JR, McMahon A, Griffin M. Perioperative management of the Fontan patient for cardiac and noncardiac Surgery. J Cardiothorac Vasc Anesth. 2022;36(1):275-85. PubMed
- John AS. Fontan repair of single ventricle physiology: Consequences of a unique physiology and possible treatment options. Cardiol Clin. 2015;33(4):559-viii. PubMed
- Ing RJ, Mclennan D, Twite MD, DiMaria M. Anesthetic considerations for Fontan-associated liver disease and the failing Fontan circuit. J Cardiothorac Vasc Anesth. 2020;34(8):2224-33. PubMed
- Jolley M, Colan SD, Rhodes J, DiNardo J. Fontan physiology revisited. Anesth Analg. 2015;121(1):172-82. PubMed
Other References
- DiNardo JA. Single ventricle physiology. OA summary. Created February 9, 2023. Accessed June 4, 2025. Link
- Sinskey J. Fontan physiology. OA-SPA Ask the Expert podcast. Created March 1, 2022. Accessed June 4, 2025. Link
- Huffmyer J. OA Intraoperative TEE Case of the Month. Created December 1, 2013. Accessed June 4, 2025. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.