Copy link
Temperature Monitoring
Last updated: 09/13/2023
Key Points
- Core temperature should be monitored in patients under general anesthesia lasting more than 30 minutes or major surgery with neuraxial anesthesia.
- The sites for monitoring core temperature include the nasopharynx, distal esophagus, tympanic membrane, and pulmonary artery.
- Axillary temperatures are most accurate when the probe is positioned over the axillary artery with the arm on the patient’s side.
Introduction
- While the core body temperature is tightly maintained within a narrow range (36.6 to 37.4°C), the temperature of the periphery varies widely depending on the environment and thermoregulatory responses.1,2 Please see the Normal Thermoregulation summary for more details.
- Temperature monitoring is an essential component of an anesthetic. The patient’s temperature should be monitored to detect changes (hypo- or hyperthermia) and to guide thermal management.
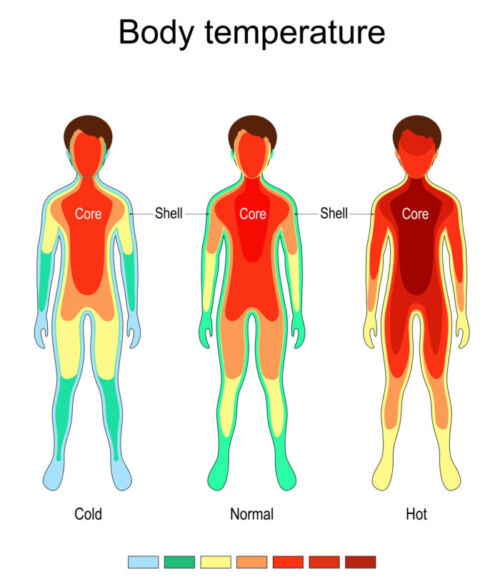
Figure 1. Central core and peripheral compartment for thermoregulation. Source: ttsz, iStockphoto.com.
When to Monitor
- The American Society of Anesthesiologists standards for monitoring states that “every patient should have temperature monitored when clinically significant changes in body temperature are intended, anticipated, or suspected.”3
- Core temperature should be monitored in patients under general anesthesia lasting more than 30 minutes or major surgery with neuraxial anesthesia.1,2
- Temperature monitoring is typically not necessary during mild or moderate sedation or when a peripheral nerve block is used.1,2
Monitoring Sites
- Whenever possible, the patient’s core temperatures should be monitored.1,2 Common sites for core temperature monitoring include:
- Nasopharynx
- Distal esophagus
- Tympanic membrane
- Pulmonary artery
- While the pulmonary artery temperature is the single best estimate of core temperature, it is rarely used.2
- Nasopharyngeal and distal esophageal temperatures are commonly used to monitor core temperature.1,2 Nasopharyngeal probes should be inserted 10-20 cm in adults to accurately estimate core temperature.1,2 Esophageal temperature probes are ideally positioned at the point of maximum heart sounds or more distally to avoid cooling by respiratory gases.2
- Tympanic membrane probes are more difficult to position correctly and are not inserted sufficiently far enough.1,2
- Alternative sites for “near-core” temperature monitoring include:
- Axilla
- Bladder
- Rectum
- Forehead
- Oral cavity
- Sublingual
- Axillary temperatures are most accurate when the probe is positioned over the axillary artery with the arm on the patient’s side.1
- Skin-surface temperatures are typically lower than core temperature.1 For example, the forehead skin temperature can be 2°C cooler than the core temperature. However, forehead temperature is less variable than other cutaneous sites.
- Rectal and bladder temperatures can be erroneous as measures of core temperature as these organs are not well-perfused to reflect changes in core heat content.1,2,4 Rectal temperatures can lag substantially. Similarly, when the urine flow is low, bladder temperatures lag behind core temperature.
Monitoring Equipment
- Commonly used thermometers in clinical use are thermistors, thermocouples, and infrared sensors.1 Most temperature probes used in anesthesia are thermistors, which are temperature-sensitive semiconductors whose electrical resistance decreases with temperatures. They are usually coated with a smooth plastic layer to allow atraumatic insertion and are labeled for single use.
- Thermocouples depend on tiny currents generated when dissimilar metals are joined.1 Most medical thermocouples use copper and constantan, a fragile copper-nickel alloy.2 Tympanic membrane probes are soft-tipped thermocouple devices that are inserted into the auditory canal to rest on the tympanic membrane. These are different from the tympanic membrane infrared scanners.
- Infrared scanners evaluate infrared energy emitted by all surfaces above absolute zero degrees.1 Temporal artery and tympanic infrared scanners are increasingly being used in recovery rooms and are not sufficiently accurate for monitoring during anesthesia.5
References
- Sessler DI. Perioperative temperature monitoring. Anesthesiology. 2021; 134(1): 111-8. PubMed
- Sessler DI. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008; 109(2): 318-38. PubMed
- American Society of Anesthesiologists standards for basic anesthetic monitoring. Accessed August 27th, 2023. Link
- Rao S, Rajan M. Heat production and loss. Update in Anaesthesia. 2008; 24(2): 182-7. Link
- Geijer H, Udumyan R, Lohse G, et al. Temperature measurements with a temporal scanner: systematic review and meta-analysis. BMJ Open. 2016; 6(3): e009509. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.