Copy link
Seizure Disorders
Last updated: 03/06/2015
Epidemiology and Risk Factors
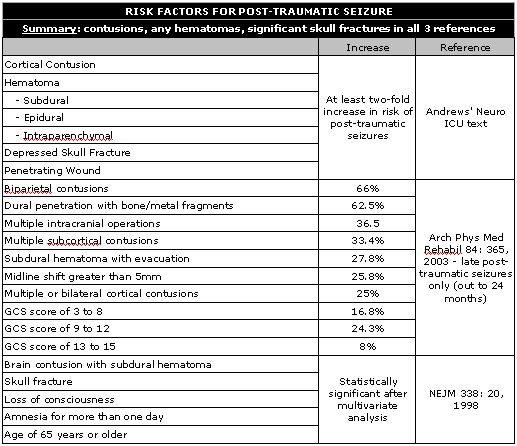
Secondary generalized seizures are the most common type seen in the ICU [Neurosurg Clin Am 4: 327, 1993]. Post-traumatic seizures are also common, either in or out of the ICU – almost 10% of the 400,000 patients treated for head injury will suffer either an early or late seizure [Neurology 30: 683, 1980]. Risk factors include cortical contusions, hematomas (especially subdural, but also epidural, or intracerebral lesions), depressed skull fracture, penetrating wound, GCS of 10 or less – if any one of these criteria are met, the incidence doubles to 20% or more. The highest cumulative probability for late posttraumatic seizures included biparietal contusions (66%), dural penetration with bone and metal fragments (62.5%), multiple intracranial operations (36.5%), multiple subcortical contusions (33.4%), subdural hematoma with evacuation (27.8%), midline shift greater than 5mm (25.8%), or multiple or bilateral cortical contusions (25%). Initial GCS score was associated with the following cumulative probabilities for development of late posttraumatic seizures at 24 months: GCS score of 3 to 8, 16.8%; GCS score of 9 to 12, 24.3%; and GCS score of 13 to 15, 8.0% [Arch Phys Med Rehabil 84: 365, 2003]. In a multivariate analysis of 4500 TBI patients, significant risk factors for later seizures were brain contusion with subdural hematoma, skull fracture, loss of consciousness or amnesia for more than one day, and an age of 65 years or older. [NEJM 338: 20, 1998]
Status Epilepticus
Status epilepticus is traditionally defined as seizures lasting 30 minutes or more, but should now include any instance of seizures lasting 5 or more minutes or two seizures with no intervening recovery [Epilepsia 40S1: S3, 1999]. The only EEG finding that seems to predict outcomes in status epilepticus is periodic epileptiform discharges, which portend a poor prognosis for recovery when present [Epilepsia 40: 157, 1999]. ALWAYS rule out hypoglycemia, hyper/hyponatremia, and other metabolic derangements. If recent lab values are unknown start thiamine and dextrose even before AEDs.
Generalized convulsive status epilepticus is the most common type and carries a mortality rate of 20-27% [Mayo Clin Proc 78: 508, 2003; NEJM 339: 792, 1998]. Non-convulsive status makes up ~ 25% of status [Chest 126: 582, 2004], is responsible for 8% of unexplained comas [Neurology 54: 340, 2000] and carries a mortality rate of 65% [NEJM 339: 792, 1998]. When EEG is used routinely, non-convulsive seizures are the most common type of seizure [Neurology 62: 1743, 2004]. Myoclonic status occurs in 33% of patients s/p out-of-hospital cardiac arrest and is a sign of devastating neurologic injury [JNNP 64: 267, 1998]
Causes of seizures in critically ill patients include sedative or opiate withdrawal (33%), metabolic abnormalities (33%), drug intoxication (15%) [Neurology 43: 1042, 1993], as well as less commonly infection, head trauma, ischemic injury, or space occupying lesions.
ALGORITHM FOR SEIZURE CONTROL
Step I
- Lorazepam 0.1 mg/kg @ 2 mg/min
Step 2 (load simultaneously)
- Phenytoin 20 mg/kg PE @ < 50 mg/min
Step 3 (for refractory cases – most require intubation)
- Propofol 3-5 mg/kg load, then 1-15 mg/kg/hr
- Midazolam 0.2 mg/kg load, 0.05 – 2 mg/kg/hr
- Pentobarbital 5-15 mg/kg load, 0.5 – 10 mg/kg/hr
(the following may not require intubation)
- Phenobarbital 20 mg/kg @ 50 mg/min
- Lidocaine 1-3 mg/kg, then continuous
Note – diazepam can be used but ALWAYS requires simultaneous phenytoin because of shorter activity [Mayo Clin Proc 78: 508; NEJM 338: 970, 1998]. Also, note that phenytoin should be dosed at 15 mg/kg in the elderly [JAMA 270: 854, 1993]. Also, total doses of 30 mg/kg can be given if initial dosing is ineffective [NEJM 338: 970, 1998] – do not give in dextrose solutions because they may cause precipitation [Clin Pharmacokin 42: 33, 2004]. Fosphenytoin may be better because it can be infused faster, does not contain propylene glycol (myocardial depression), is compatible with dextrose, and doesn’t cause skin necrosis [Clin Pharmacokin 42: 33, 2004]. Phenobarbital is the most effective agent available for non-convulsive seizures [NEJM 339: 792, 1998] but can cause hypotension, respiratory depression, and prolonged sedation. Beware “anticonvulsant hypersensitivity syndrome” in 1:1000 patients – triad of fever, rash, and lymphadenopathy to phenytoin or phenobarb (cross reactivity 50%, withdraw medication and give diazepam [Ann Pharmacother 27: 298, 1993]). As last ditch effort, try propofol, midazolam, or pentobarbital along with intubation.
Propofol has been used successfully in the treatment of refractory status epilepticus, both in adults and children [Epilepsia 41: 105, 2000]. In a study of 16 patients with refractory status epilepticus, propofol achieved seizure control in 2.6 minutes on average, as opposed to 123 minutes for phenobarbital (p = 0.002) [Epilepsia 39: 18, 1998]. Propofol is loaded at 1-2 mg/kg, then given at 2-10 mg/kg/kr titrated to either burst suppression or reduction in clinical seizure activity, maintained for 24 hours after which point withdrawal is begun.
Lastly, there are many case reports describing the use of lidocaine in both adult and neonatal literature – initial lidocaine doses used to stop seizures have ranged from 1 to 3 mg/kg. Most reports recommend a maintenance infusion of lidocaine after initial termination of SE, and a continuous infusion is almost universally recommended for neonates. Toxicity from a 1.5-2.0 mg/kg dose of lidocaine for the control of SE is rare. [Acad Emerg Med. 4: 918, 1997]
Seizure Prophylaxis after Trauma
Phenytoin has been shown to reduce the incidence of early post-traumatic seizures – the Temkin study, a trial of 404 patients randomized to phenytoin vs. placebo showed reduction in the first week from 14.2 to 3.6% (p = 0.001) [NEJM 323: 497, 1990], but no change from days 8-235 [NEJM 323: 497, 1990; JNNP 64: 108, 1998]. Phenytoin has some unfortunate neurobehavioral side effects, however as of now there is no alternative. In a study of over 300 patients by Temkin, valproate for 1 or 12 months in post-traumatic head injury patients also reduced post-traumatic seizures, however there was also a trend towards increased mortality (p = 0.07) [J Neurosurg 91: 593, 1999] (note that the different treatment duration made this study difficult to interpret).
Summary of data on prophylactic AEDs s/p Trauma
The Temkin data (404 randomized patients) supports 7 days of phenytoin (one week risk of seizures reduced from 14.2 to 3.6%, p = 0.001) [NEJM 323: 497, 1990]
There is no benefit of giving AEDs longer than 7 days [NEJM 323: 497, 1990; JNNP 64: 108, 1998]
Seizure Prophylaxis for Newly-Diagnosed Brain Tumors (NOT post-operative)
20% of patients with a newly diagnosed brain tumor will have already suffered a seizure. Another 19% will suffer a seizure after the diagnosis. However, a metaanalysis of class I and II data published by the Quality Standards Subcommittee of the American Academy of Neurology showed no significant reduction in first seizures in patients undergoing intracranial surgery for brain tumors. [Neurology 54: 1886, 2000]
Summary of data on prophylactic AEDs in new brain tumor patients
The Quality Standards Subcommittee of the American Academy of Neurology metaanalysis showed no significant reduction in first seizures in patients undergoing intracranial surgery for brain tumors. [Neurology 54: 1886, 2000]
Seizure Prophylaxis after Craniotomy
In a double-blind trial, epilepsy was observed in 7.9% (8/101) of patients treated with phenytoin and in 16.7% (17/102) of those receiving placebo (75% occurred within a month of surgery). Therapeutic drug levels were associated with a significant reduction in the frequency of epilepsy. High rates of epilepsy have been observed after cranial surgery in patients with meningioma, aneurysm, and head injury with or without intracranial clots, and routine prophylaxis with phenytoin would seem to be indicated in such patients [Lancet 23: 384, 1980] In another double-blind trial of phenytoin following craniotomy, a significant reduction in the frequency of epilepsy was observed in the group receiving the active drug up to the 10th postoperative week (two-thirds of seizures occurred within 1 month of surgery). High rates of epilepsy were observed after surgery in patients with meningioma, metastasis, aneurysm, and head injury. Routine prophylaxis with phenytoin (in a dosage of 5 to 6 mg/kg/day) would seem to be indicated, particularly in high-risk patients and, where possible, this treatment should be started 1 week preoperatively [J Neurosurg 58: 672, 1983]
In another randomized, double-blind study, 15 mg/kg IV phenytoin was given to 189 patients shortly before intracranial, supratentorial surgery, followed by 5-6 mg/kg/day IV in three divided doses x 3 days. Levels of 10-20 micrograms/mL were achieved in 59.8% patients. 185 randomized controls received a placebo. The phenytoin group had one immediate and two early postoperative seizures, compared to four immediate and nine early postoperative seizures. Also, to avoid a decrease in the anticonvulsant due to blood loss, the authors recommend giving AEDs at least 20 minutes before completion of wound closure [Surg Neurol 31: 361, 1989]
On the other hand, Foy and Chadwick studied carbamazepine vs. phenytoin vs. no treatment in 276 patients randomised to treatment for six or 24 months – although confidence limits, were large, the results showed no significant differences between the regimes in terms of seizures or death. A high incidence of drug-related side effects was found in the treatment groups, prophylactic anticonvulsants were therefore not recommended routinely following supratentorial craniotomy [J Neurol Neurosurg Psychiatry 55: 753, 1992]
A prospective, stratified, randomised, double blind single centre clinical trial compared two groups of 50 craniotomy patients who were treated for 1 year after surgery with either 300 mg phenytoin/day or 1500 mg sodium valproate/day showed that in each group, 7 patients experienced one or more postoperative seizures. In all patients, drug plasma concentrations were in the low or subtherapeutic ranges at the time of the first postoperative seizure. No major differences were found between phenytoin and valproate prophylaxis [J Neurol Neurosurg Psychiatry 67: 474, 1999] A metaanalysis of the available literature found 30 studies on the subject, only 6 of which were controlled and three of which were of satisfactory methodological quality. The summation of those three studies suggested that prophylactically used AEDs prevented postoperative convulsions, but this effect was not statistically significant (p = 0.1 one-tailed) [Seizure 5: 291, 1996] 200 patients with supratentorial brain tumors already taking an AED were randomized – one group received PHY (18 mg/kg intraoperative, followed by daily doses aimed maintaining 10-20 aeg/ml) for 7 days. More than 90% of patients continued to take preexisting AEDs (carbamazepine or Phenobarbital). 13% of the additional phenytoin and 11% of the placebo group (p > 0.05) had seizures. Prophylactic administration of PHT cannot be recommended in patients treated with concomitant AEDs [Epilepsia 43: 175, 2002]
Summary of data on prophylactic AEDs s/p craniotomy
- 3 RCTs totaling over 600 patients from the 1980’s support phenytoin after craniotomy
- North’s data show benefits as far out as 10 weeks post-operatively [J Neurosurg 58: 672, 1983]
- A more recent study of 276 patients showed no difference between phenytoin, carbamazepine, and no treatment [J Neurol Neurosurg Psychiatry 55: 753, 1992]
- A meta-analysis on the subject suggested a benefit of AEDs (p = 0.10) [Seizure 5: 291, 1996]
- Phenytoin and valproate appear to be equivalent [J Neurol Neurosurg Psychiatry 67: 474, 1999]
- There is no benefit to adding phenytoin to carbamazepine or Phenobarbital in patients already taking AEDs prior to surgery
- 2/3 to 3/4 of post-craniotomy seizures will occur within one month of surgery
- Meningioma, aneurysm, metastasis, and head injury are associated with a high likelihood of postoperative epilepsy
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.