Copy link
Patent Ductus Arteriosus
Last updated: 04/04/2023
Key Points
- Patent ductus arteriosus (PDA) is the failure of the ductus arteriosus, a fetal shunt connecting the proximal descending aorta to the pulmonary artery, to close completely in the postnatal period.1
- PDA can lead to systemic hypoperfusion and pulmonary overcirculation in infants and, if left untreated, can cause congestive heart failure, pulmonary hypertension, and infective endocarditis in older children and adults.2
- Anesthetic considerations for surgical repair include the location of the operation, surgical vs. percutaneous transcatheter closure, characteristics of the shunt, and close hemodynamic monitoring.2,3
Anatomy
- PDA is formed from the distal portion of the left sixth pair of the embryonic aortic arch known as the ductus arteriosus – this connection usually degenerates by the 8th week of life.1
- PDA is a connection between the descending aorta distal to the left subclavian and the left pulmonary artery, which has failed to completely close postnatally.1
- The variability of PDA size impacts the degree of shunting.
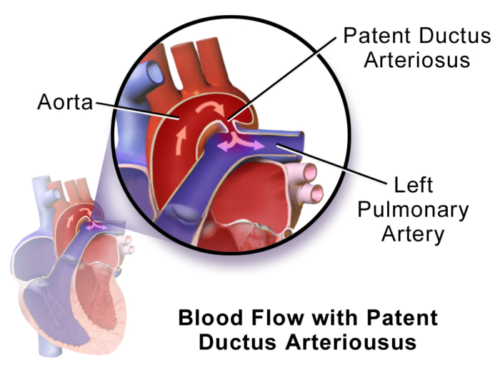
Figure 1. Patent ductus arteriosus. Blood flowing from aorta to pulmonary artery in a left-to-right shunt. Source: Wikimedia. By BruceBlaus. CC BY 3.0, Link.
Pathophysiology
- The ductus arteriosus allows fetal circulation to bypass the lung’s high-resistance system, allowing blood to flow from the pulmonary artery through the ductus arteriosus to the descending aorta and the rest of the body.1
- Patency: The ductus arteriosus remains patent due to low fetal oxygen tension, prostaglandin (PGE2), and prostacyclin (PGI2). Prostaglandin and prostacyclin are produced by the placenta and have decreased metabolization in fetal lungs leading to high concentrations in fetal circulation.1
- Closure: After birth, an increase in arterial oxygen tension and decreases in circulating prostaglandins, pulmonary vascular resistance, and ductal blood flow reverse direction of the shunt and instigate closure of the ductus arteriosus.
- Ductus arteriosus turns into ligamentum arteriosum (a fibrous band with no lumen) – functionally closes at 24-48 hours and physically closes in 2-3 weeks via fibrosis.1
- PDA occurs when the ductus arteriosus does not involute and instead remains open. Etiologies include prematurity, maternal exposures during pregnancy (e.g., rubella, alcohol consumption, phenytoin use), prostaglandin administration, and genetic anomalies (e.g., trisomies).
- PDA flow and shunt direction are determined by the gradient between the systemic and pulmonary vascular resistances as well as anatomic features of the duct (length, diameter, and tortuosity).
- Chronic exposure to high volumes via shunt leads to left atrial dilation and congestive heart failure due to increased volume load to the left ventricle. Over time, increased pulmonary artery flow leads to increased pulmonary vascular resistance and pulmonary hypertension. Severe pulmonary hypertension can reverse the left-to-right shunt leading to Eisenmenger syndrome.1
- In older patients, PDA calcification and aneurysmal dilation increase the risk of rupture.
Management
- Asymptomatic, small PDAs with no evidence of volume overload can be observed carefully.
- If symptomatic, PDA is initially treated with digoxin and furosemide.4
- Elective ductal closure is considered if there is left heart enlargement, mild to moderate pulmonary hypertension, or symptomatic PDA with signs of volume overload or increased work of breathing.4,5
Pharmacologic Intervention
- Premature infants: pharmacologic intervention with indomethacin or ibuprofen is preferred initially to stimulate duct closure. For patients in whom indomethacin or ibuprofen is contraindicated (bleeding, thrombocytopenia, hyperbilirubinemia, and/or concern for necrotizing enterocolitis or cerebral ischemia, etc.) acetaminophen may also be used for PDA closure.
- Term infants: not recommended due to decreased effectiveness
Transcatheter (TC) Closure
- In general, TC closure is preferred over surgical closure in all age groups.
- In the past, interventional closure was reserved for infants that were sufficiently sized to minimize procedural complications. Patients who could not be delayed to allow for growth were managed surgically.1
- Recently, TC closure devices have been shown to be safe in patients as small as 700gm.6
Surgical Closure
- Surgical closure is indicated when there is anatomy that is nonconducive to TC closure as well as when size-appropriate devices and/or procedural expertise are lacking.
- For a left-sided PDA, the typical approach is through a left posterior thoracotomy via an extrapleural approach. In larger patients, a video-assisted thoracoscopic technique (VATS) may be used.
Anesthetic Considerations
Preoperative Evaluation
- The patient’s past medical history should be reviewed. For premature neonates, particular attention should be paid to ventilator settings and FiO2 requirement.
- Clinicians should determine whether the PDA ligation will be done via thoracotomy, VATS, or TC device closure.2
- The echocardiogram should be reviewed to assess for PDA location, size, and patency as well as ventricular function and concomitant cardiac lesions.
- The need for single-lung ventilation will depend on surgical technique and patient size.
- Adequate vascular access should be established.
- Correct antibiotic prophylaxis should be determined to prevent subacute bacterial endocarditis.
Goals
- Substantial increase or decrease in pulmonary vascular resistance should be avoided to prevent right-to-left shunting and maintain systemic blood flow.3
- Heart rate, contractility, and preload should be preserved to maintain cardiac output.3
- Preductal and postductal pulse oximetry and blood pressure should be established in the upper and lower extremities. Arterial lines are usually unnecessary.2
- Clinicians should determine whether the correct vessel has been ligated by monitoring distal pulse oximetry saturation and perfusion during test clamping of the PDA.2
- Normothermia throughout periprocedural period should be maintained.
Induction
- High-dose fentanyl is the anesthetic of choice in neonates as it provides adequate analgesia and stable hemodynamics. It may be used in combination with a benzodiazepine.2
- Mask induction with cautious titration of sevoflurane (4-6%) is generally well-tolerated in older patients. The administration of a premedication may decrease the amount of volatile required. This method is poorly tolerated in premature infants and patients with congestive heart failure.
- FiO2 should be decreased once the patient is safely intubated in order to decrease the risk of systemic hypoperfusion and pulmonary overcirculation. This is especially important in patients with large, hemodynamically significant PDAs.
Maintenance
- General anesthesia may be maintained with low-dose volatile agents and boluses of short-acting opioids (fentanyl) and a neuromuscular blocking agent.2,3 A remifentanil infusion (0.5-1.0 μgkg–1 min– 1) can also be utilized.3
- During maintenance, an O2 saturation appropriate for the patient should be maintained with the lowest FiO2 possible. The ability to use a low FiO2 may be limited by an increased oxygen requirement due to left lung retraction during surgery.2
- Regional anesthesia with erector spinae blocks or paravertebral blocks may be considered in selected patients.
Postoperative Considerations
- Pain management: Acetaminophen and intermittent doses of opioids are usually sufficient.
Complications Postrepair
- Recurrent laryngeal nerve injury leading to vocal cord paresis or paralysis;2 the left recurrent laryngeal nerve arises from the left vagus nerve in front of the aortic arch and is in close proximity to the ductus arteriosus (Figure 2). Postoperative stridor or hoarseness should raise the suspicion for possible recurrent laryngeal nerve injury.
- Inadvertant ligation of the left pulmonary artery or descending aorta
- Pneumothorax
- Postligation cardiac syndrome: hypotension requiring inotropic support with failure of oxygenation and ventilation due to left ventricular systolic and diastolic failure in the setting of acute changes in afterload.7
Figure 2. Recurrent laryngeal nerve located near PDA. Source: Wikimedia. Patrick J. Lynch CC BY 2.5, Link.
References
- Schneider DJ, Moore JW. Patent ductus arteriosus. Circulation. 2006;114(17):1873-1882. PubMed
- Kritzmire SM, Boyer TJ, and Singh P. Anesthesia For patients with patent ductus arteriosus. In: StatPearls (Internet). Treasure Island, FL. 2022. Accessed December 8, 2022. PubMed
- Nasr VD, DiNardo JA. Patent ductus arteriosus. In: The Pediatric Cardiac Anesthesia Handbook, First Edition. Viviane G. Nasr VG, DiNardo JA (eds). Hoboken, NJ. John Wiley & Sons Ltd. 2017:67-71.
- Doyle T, Kavanaugh-McHugh A, Soslow J. Management of patent ductus arteriosus in term infants, children, and adults. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. Accessed on December 8, 2022. Link
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Intervention. J Am Coll Cardiol. 2008;52(23). PubMed
- Sathanandam SK, Gutfinger D, O'Brien L, et al. Amplatzer Piccolo occluder clinical trial for percutaneous closure of the patent ductus arteriosus in patients ≥700 grams. Catheter Cardiovasc Interv. 2020;96(6):1266-76. PubMed
- Giesinger RE, Bischoff AR, McNamara PJ. Anticipatory perioperative management for patent ductus arteriosus surgery: Understanding postligation cardiac syndrome. Congenit Heart Dis. 2019;14(2):311-6. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.