Copy link
Neuromuscular Blockade: Effect of Drugs and Medical Conditions
Last updated: 05/09/2023
Key Points
- Neuromuscular blocking agents (NMBA) can be affected by drug interactions and various neuromuscular disease states.
- NMBAs should be used with caution in patients with neuromuscular diseases such as myasthenia gravis and muscular dystrophy.
Anesthetic Agents
Inhalational Agents
- Inhalational agents potentiate neuromuscular blockade in a dose-dependent fashion and act at the neuromuscular junction (NMJ).
- At a similar minimum alveolar concentration, the impact on NMB is as follows: desflurane > sevoflurane > isoflurane > nitrous oxide.
- The effects of inhaled agents on neuromuscular blockade occur in a concentration-dependent fashion.2
- Nitrous oxide has either little to no effect on neuromuscular blockade.1,2
Intravenous Agents
- Local anesthetics produce effects at the NMJ by themselves and potentiate neuromuscular blockade.
- When multiple muscle relaxants are used, they can have synergistic, additive, or antagonistic effects.
- Depending on the mechanism of action, the combination of two nondepolarizing agents is synergistic or additive.
- An antagonistic effect is obtained when a nondepolarizing agent is administered prior to a depolarizing agent to prevent fasciculations and myalgias.1,2
Antibiotics
- The aminoglycosides (neomycin, gentamicin, tobramycin) and the lincosamides (clindamycin) potentiate neuromuscular blockade, although generally, not at clinically significant doses.
- Commonly used antibiotics like penicillins, cephalosporins, and nitroimidazoles do not have any effects on neuromuscular blockade.1,2
Anticonvulsants
- Chronic anticonvulsant therapy causes resistance to neuromuscular blockade via pharmacodynamic and pharmacokinetic mechanisms.
- Acute administration of some anticonvulsants (phenytoin, carbamazepine) can potentiate blockade at the NMJ.1,2
Other Drug Interactions
- Drugs that alter cardiac output can alter the response to NMBAs. For example, drugs that increase chronotropy can accelerate NMBA action, while drugs that decrease chronotropy can delay NMBA onset.
- Cations, such as magnesium, and drugs that resemble (lithium) antagonize calcium’s effects at the NMJ as well as prolong NMBA action.1,2
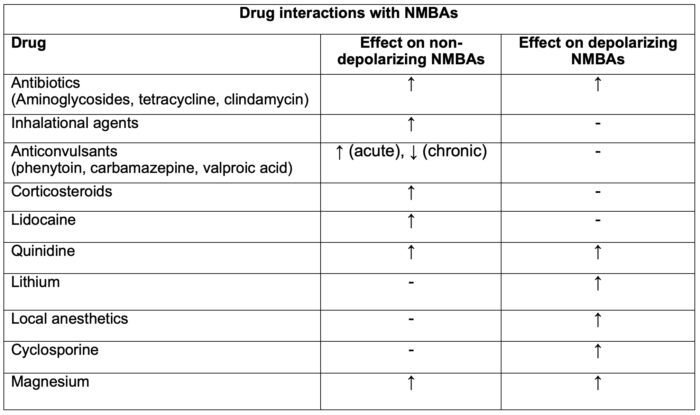
Table 1. Drug interactions with NMBAs. Adapted from Faulk DJ. Neuromuscular blockade and anticholinesterases. In: Chu LF, Traynor AJ, Kurup V. Manual of Clinical Anesthesiology. Philadelphia, PA. 2nd edition. Wolters Kluwer; 2020: 223-231.
Legend: ↑ = enhancement or prolongation of blockade, - = no effect, ↓ = inhibition or decreased duration of blockade.
Effects of Medical Conditions on NMBAs
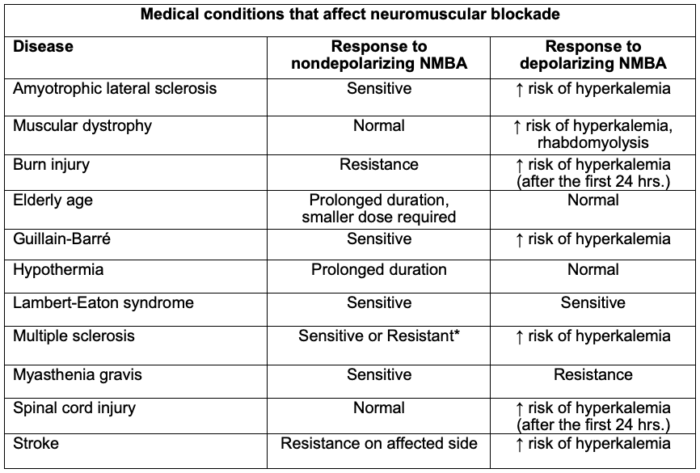
Table 2. Medical conditions that affect neuromuscular blockade. The degree to which the above-mentioned drugs and physiologic states interfere with NMB is clinically variable; thus, qualitative monitoring of neuromuscular blockade is recommended. *Can be sensitive or resistant depending on the degree of muscle wasting and medication management. Adapted from Faulk DJ. Neuromuscular blockade and anticholinesterases. In: Chu LF, Traynor AJ, Kurup V. Manual of Clinical Anesthesiology. Philadelphia, PA. 2nd edition. Wolters Kluwer; 2020: 223-231.
Myasthenia Gravis (MG)
- Myasthenia gravis results in a decrease in the postsynaptic acetylcholine receptors (AChRs) in the NMJ. Patients with MG are extremely sensitive to the neuromuscular depressant effects of inhaled anesthetics and also to nondepolarizing agents. Nondepolarizing NMBAs (rocuronium and vecuronium) are preferred, and they should be administered in small, incremental doses with frequent blockade monitoring.
- Patients with MG are resistant to depolarizing NMBAs, which is thought to be due to decreased amount of acetylcholine (Ach) receptors at the post-synaptic AChRs.
- Sugammadex is preferred for the reversal of neuromuscular blockade. If necessary to use anticholinesterase drugs, slow titration is recommended to obtain antagonism and avoid a cholinergic crisis.3,4
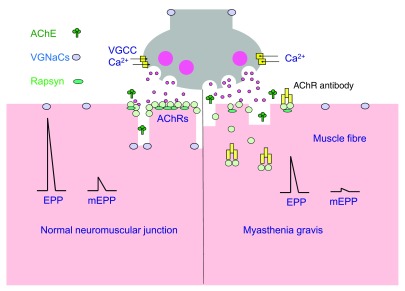
Figure 1. Neuromuscular transmission in patients with myasthenia gravis. Source: Phillips WD, Vincent A. Pathogenesis of myasthenia gravis: update on disease types, models, and mechanisms. F1000Res. 2016;5: F1000 Faculty Rev-1513. CC BY SA PubMed.
Myotonic Dystrophy (Myotonia)
- Myotonic dystrophy is characterized by delayed relaxation of skeletal muscle after contraction due to ion channel abnormalities in muscle membranes.
- If muscle relaxation is needed, the use of nondepolarizing agents is preferred with deliberate neuromuscular blockade monitoring to prevent myotonia from the muscle stimulation.
- Succinylcholine should be avoided in these patients as the effect can be unpredictable, leading to exaggerated contracture and a difficult to ventilate and difficult to intubate scenario. Electrolyte imbalances such as hyperkalemia can be dangerous in these patients.3,4
Muscular Dystrophy
- Muscular dystrophies are disorders that cause progressive weakness and impact cardiac, smooth, and skeletal muscles (i.e., Duchenne, Becker, Limb-Girdle). The underlying pathophysiology of these diseases is abnormal or inadequate muscle membrane proteins.
- Succinylcholine is contraindicated due to an increased risk of rhabdomyolysis and hyperkalemia.
- If neuromuscular blockade is required, the use of rocuronium with sugammadex reversal is preferred.3,4
Upper Motor Neuron Lesions
- Upper motor neurons (UMN) span from the cerebral cortex to the spinal cord and control voluntary muscle movements. Injury to UMNs can occur from stroke, anoxic/traumatic brain injury, malignancy, or neurodegenerative disorders (i.e., multiple sclerosis, amyotrophic lateral sclerosis).
- Injury to UMNs is characterized by upper motor neuron syndrome consisting of symptoms such as spasticity, hyperreflexia, clonus, and weakness.
- Cautious use of nondepolarizing NMBAs is recommended due to the increased sensitivity to their effects and the risk of perioperative respiratory complications.
- Succinylcholine is not recommended in these patients due to an increased risk of hyperkalemia from denervated muscles, placing these patients at increased risk for cardiac arrhythmias.3,4
Renal Failure
- The majority of the nondepolarizing NMBs are hydrophilic due to a quaternary ammonium ion. In renal failure, clearance and elimination of NMBs can be impaired. Atracurium and Cisatracurium metabolism and clearance is independent of renal function.
- Depolarizing agents are metabolized by plasma cholinesterases which may decreased in renal failure.4
Liver Failure
- There is a range of pharmacokinetics and pharmacodynamics in liver failure due to varying effects on volume of distribution, metabolism, biliary excretion, and esterase synthesis.4
Other Conditions
- Special attention should be given to patients who have suffered denervation (burns) and have associated upregulation of nicotinic acetylcholine receptors, as they are at a high risk of life-threatening hyperkalemia with the administration of succinylcholine.
- Both total body weight and ideal body weight are used for the dosing of NMBAs in obese patients. Dosing of nondepolarizing agents is often based upon ideal body weight to prevent prolonged paralysis. Dosing of succinylcholine should be based upon total body weight secondary to an increased volume of distribution and increased pseudocholinesterase activity.
- Patient factors that impact the volume of distribution (extremes of age) and metabolism (liver or kidney disease) can impact the loading and maintenance doses of NMBAs, and often require close neuromuscular monitoring intraoperatively.
- Hypothermia prolongs the duration of action of nondepolarizing NMBs by affecting plasma clearance, renal and hepatic excretion, volume of distribution and receptor affinity.3,4
References
- Jeevendra Martyn JA, Fagerlund MJ. Neuromuscular physiology and pharmacology. In: Gropper M, Cohen NH, Miller RD, et al.(eds) Miller’s Anesthesia. Philadelphia, PA. 9th edition. Elsevier; 2019:333-353.
- Brull SJ, Meistelman C. Pharmacology of Neuromuscular Blocking Drugs. In: Gropper MA, Cohen NH, Miller RD, et al.(eds) Miller’s Anesthesia. Philadelphia, PA. 9th edition. Elsevier; 2019: 792-831.
- Faulk DJ. Neuromuscular blockade and anticholinesterases. In: Chu LF, Traynor AJ, Kurup V. Manual of Clinical Anesthesiology. Philadelphia, PA. 2nd edition. Wolters Kluwer; 2020: 223-231.
- Renew JR. Clinical use of neuromuscular blocking agents in anesthesia. In: Post T, ed. UpToDate. 2023 Accessed April 10th, 2023. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.