Copy link
Trisomy 18 and 13
Last updated: 05/02/2025
Key Points
- Trisomy 18 (Edwards syndrome) and trisomy 13 (Patau syndrome) are caused by an extra copy of the long arm of chromosomes 18 and 13, respectively; this can result from complete, partial, or mosaic trisomy.
- The clinical features of trisomy 18 include rocker-bottom feet, clenched fists with overlapping fingers, prominent occiput, microcephaly, micrognathia, intellectual disability, cardiac defects, and omphalocele.
- The clinical features of trisomy 13 include microphthalmia, holoprosencephaly, microcephaly, cleft lip/palate, polydactyly, rocker-bottom feet, and intellectual disability.
- Perioperative anesthetic goals include preparation for a potentially difficult airway, maintaining stable hemodynamics, and preventing seizures.
Trisomy 18
Epidemiology
- Trisomy 18 or Edwards syndrome is the second most common autosomal trisomy.1
- In the US, the prevalence is approximately 1 in 8600 live births.2
- Like other trisomy syndromes, trisomy 18 is associated with advanced maternal age. In the last 2 decades, average maternal age has increased, resulting in an increased overall prevalence of trisomy 18. Notably, the prevalence in live births has decreased given advancements in prenatal diagnosis and subsequent pregnancy termination in 86% of cases.3,4
- The prevalence in females is higher than in males by a ratio of 3:2 and affected females tend to have better survival rates than males.2
Etiology
- Edwards syndrome is caused by an extra copy of the long arm of chromosome 18, 18q.2
- Trisomy of the short arm of chromosome 18 (18p) is not associated with Edwards syndrome.2
- Three genetic etiologies can result in this extra chromosome: complete trisomy, partial trisomy, and mosaic trisomy.2
- The most common type of trisomy 18, accounting for 94% of cases, is complete trisomy with three full copies of chromosome 18. This results from meiotic nondisjunction during meiosis II. The nondisjunction event is most often a maternal event, and these errors occur more frequently with advanced maternal age.2
- The second most common type of trisomy 18, accounting for less than 5% of cases, is mosaicism. Both a complete trisomy 18 cell line and a normal cell line exist in mosaicism. This is caused by a mitotic nondisjunction error unrelated to maternal age. The associated phenotype can range from a complete trisomy 18 phenotype to a normal phenotype.2,4
- The least common type of trisomy 18, accounting for 2% of cases, is partial trisomy with a segment of chromosome 18’s long arm present in triplicate. This can occur due to a translocation or inversion carried by either parent. The resulting phenotype can vary depending on the location and extent of the triplicated segment.2,3
Screening: Labs and Ultrasound1-3
- Prenatal maternal labs can show nonspecific but abnormal findings such as low alpha-fetoprotein levels, human chorionic gonadotropin, and unconjugated estriol.2
- Routine prenatal ultrasounds may show abnormal sonographic findings such as:
- Intrauterine growth restriction
- Polyhydramnios
- Clenched hands
- Choroid plexus cysts
- Agenesis of the corpus callosum
- Nuchal thickening
- Single umbilical artery
- Brachycephaly
- Cardiac defects
- Omphalocele
Diagnosis2
- Diagnosis can be confirmed prenatally via prenatal genetic analysis; options include chorionic villus sampling, amniocentesis, and fetal cell-free DNA analysis.
- Postnatal diagnosis is usually clinical but can be confirmed with chromosome studies such as karyotyping or microarray testing.
Clinical Manifestations1-4
- The clinical manifestations of Edwards syndrome are variable and usually involve multiple organ systems. There is no single clinical feature that is pathognomonic for Edwards syndrome.
- Clinical features can include the following:
- Intrauterine growth restriction, low birth weight
- Craniofacial abnormalities: prominent occiput, microcephaly, micrognathia, triangular/asymmetric face, facial paralysis, dysplastic ears, small ears, preauricular appendages, microphthalmia, widely spaced eyes, epicanthic folds, short palpebral fissures, upward or downward slanting palpebral fissures, prominent nasal bridge with hypoplastic nasal root, choanal atresia, small mouth, cleft lip, cleft palate, narrow arched palate
- Skeletal abnormalities: growth retardation, short neck, short sternum, small pelvis, scoliosis, broad chest, incomplete ossification of clavicle, polydactyly, syndactyly
- Extremity abnormalities: clenched hands with index finger overlapping the third finger and the fifth finger overlapping the fourth finger, single palmar crease, thumb hypoplasia, hypoplastic nails, rocker-bottom feet, prominent calcaneus, clubfoot, dorsiflexed great toes
- Congenital heart disease: valvular abnormalities, ventricular septal defect, atrial septal defect, patent ductus arteriosus, tetralogy of Fallot, overriding aorta, coarctation of the aorta, and hypoplastic left heart
- Pulmonary abnormalities: pulmonary hypoplasia, tracheobronchomalacia, laryngomalacia, obstructive and central apnea, and pulmonary hypertension
- Gastrointestinal abnormalities: Meckel diverticulum, tracheaesophageal fistula, pyloric stenosis, intestinal malrotation, esophageal atresia, and omphalocele
- Renal/genital abnormalities: horseshoe kidney, renal agenesis, hydronephrosis, hypospadias, cryptorchidism, clitoral hypertrophy, hypoplasia of the labia majora, ovarian dysgenesis, and bifid uterus
- Neurologic: neonatal hypotonia followed by hypertonia, apnea, seizures, mental retardation, poor sucking, cerebellar hypoplasia, meningoencephalocele, anencephaly, holoprosencephaly, Arnold-Chiari malformation, hypoplasia of corpus callosum, hydrocephaly, severe intellectual disability, and developmental delays
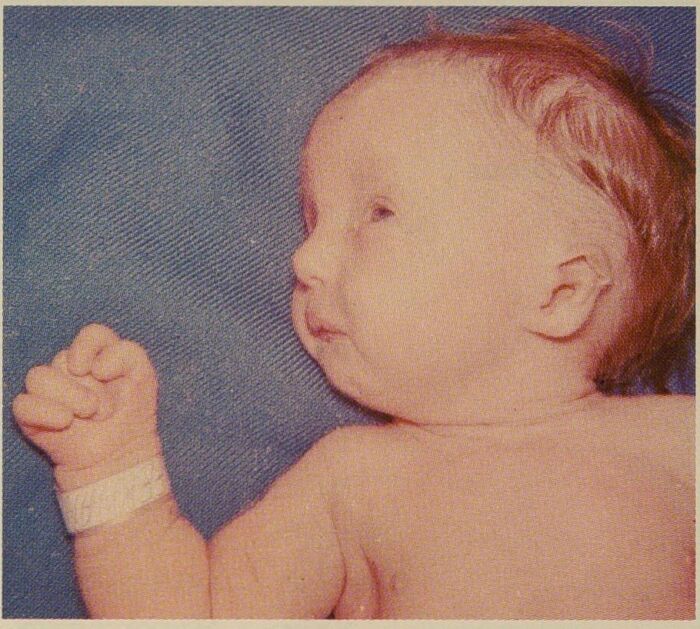
Figure 1. Patient with trisomy 18 showing micrognathia, clenched fist, and overlapping fingers. Source: Wikipedia. Sydney Gellis and Murray Feingold. Public Domain. Link
Life Expectancy and Prognosis
- Most patients with trisomy 18 die in utero or during birth. As gestational age increases, the likelihood of survival to term also increases.3
- For those that survive through birth, the median survival ranges from 3-14.5 days. Only 5-10% of patients survive past the first year of life.1,2
- The major causes of death in these patients are cardiac failure and respiratory failure.2
- Studies have shown that female patients tend to survive longer than their male counterparts.4
- More recently, several reports of longer survival due to aggressive medical therapy have emerged, although these children live with severe disabilities.1
Trisomy 13
Epidemiology1
- Trisomy 13 or Patau syndrome occurs in 1 out of 5000 births. Like the other trisomy syndromes, it is associated with advanced maternal age.
Etiology
- Patau syndrome is caused by an extra copy of chromosome 13.1
- Similar to trisomy 18 as discussed above, the three genetic etiologies thought to cause trisomy 13 are complete, partial and mosaic trisomy.5
Screening5
- Abnormal findings associated with Patau syndrome can be seen on routine prenatal screening ultrasounds.
- There is an increase in the sensitivity of ultrasound as a screening tool after 17 weeks of gestation.
- Typical findings on prenatal ultrasound include:
- Holoprosencephaly
- Neural tube defects
- Midline facial abnormalities
- Cardiac, GI, renal anomalies
- Intrauterine growth restriction
Diagnosis5
- Prenatally, diagnosis of Patau syndrome can be made with chorionic villi sampling, amniocentesis, or fetal cell-free DNA analysis.
- After birth, diagnosis can be made clinically or confirmed with chromosome studies such as karyotyping.
Clinical Manifestations1,5
- The clinical manifestations of Patau syndrome are variable and can involve multiple organ systems, as below:
- Craniofacial: microcephaly, midline defects such as cyclopia, cleft lip, and cleft palate, small malformed ears, anophthalmia, microphthalmia, sloping forehead, narrow palate, micrognathia, and cutis aplasia
- Central nervous system: holoprosencephaly, seizures, and deafness
- Extremities: polydactyly, club feet, rocker-bottom feet, capillary hemangiomata, simian crease, syndactyly, hyperconvex fingernails, and retroflexible thumb
- Congenital heart disease: ventricular septal defect, atrial septal defect, tetralogy of Fallot, double origin right ventricle, patent ductus arteriosus, dextrocardia, etc.
- Genitourinary: cryptorchidism, hypospadias, labia minora hypoplasia, bicornuate uterus, polycystic kidney disease, hydronephrosis, horseshoe kidney
- Gastrointestinal: omphalocele, incomplete rotation of colon, Meckel diverticulum
- Intrauterine growth restriction, developmental delays, psychomotor disorder, intellectual disability, and failure to thrive
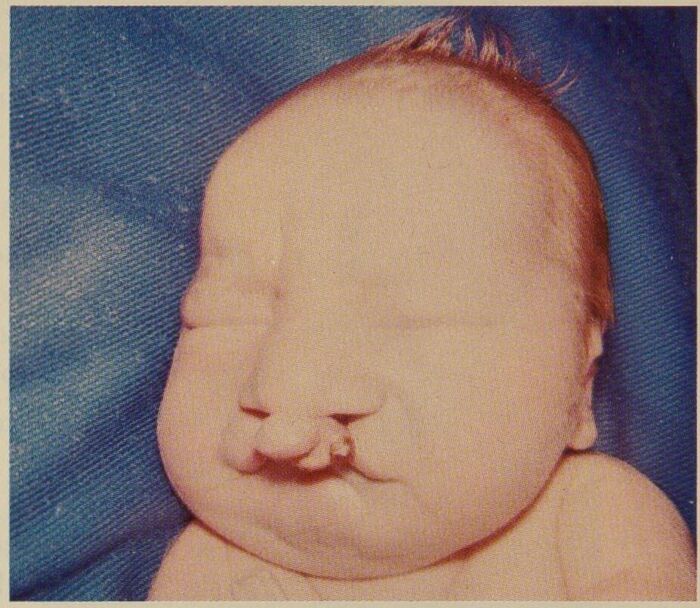
Figure 2. Patient with trisomy 13 showing cleft lip. Source: Wikipedia. Syndey Gellis and Murray Feingold. Public Domain. Link
Life Expectancy and Prognosis
- Most patients with trisomy 13 die in utero.1,5
- The median survival for those who survive until birth is 7 to 10 days. About 80 percent die within the first month of life, and 90% die within the first year of life.1,5
- Those who survive longer tend to have lower rates of cardiac defects than the overall patient population.1
- Intensive medical and surgical therapy may prolong survival, but is controversial due to poor prognosis, even with aggressive treatment.1,5
Anesthetic Considerations for Patients With Trisomy 13 or 18
Need for Anesthesia
- Many children with Edwards and Patau syndrome undergo surgical procedures for therapeutic or palliative purposes and thus require anesthetic management.7
- The perioperative management of these patients should be approached using a multidisciplinary approach, emphasizing preoperative planning.
- Given the variability in clinical manifestations associated with these syndromes, a definitive universal anesthetic protocol for these syndromes does not exist.8
- Anesthetic plans must be precisely crafted on a case-by-case basis to cater to each patient’s unique disease presentation.
Cardiac Workup
- A preoperative cardiac workup should be completed, as trisomy 13 and 18 are known to have associated congenital heart defects. This may include a thorough physical examination, echocardiogram, electrocardiogram, chest x-ray, and possible evaluation by a pediatric cardiologist for medical optimization.7
- In some cases, antibiotic prophylaxis for endocarditis may be indicated.9
Airway Management
- A thorough preoperative airway exam should be conducted, as patients with trisomy 13 or 18 can have multiple risk factors for difficult mask ventilation and intubation, such as micrognathia, small mouth openings, high arched palates, nasal malformations, and short necks, among other airway abnormalities.6,7
- Intubating laryngeal mask airways, video laryngoscopy, and fiberoptic laryngoscopy should be readily available prior to induction of anesthesia.7
- In some cases, the patient’s mental status may limit their ability to participate in a complete preoperative airway exam. In such situations, a slow inhalational induction with maintenance of spontaneous ventilation may be employed to allow the anesthesiologist to perform a more complete airway exam after induction prior to muscle relaxation and intubation.8,9
- In contrast, a rapid sequence intubation may be a safer option for patients with anomalies of the upper GI tract predisposing them to aspiration.6
Intraoperative Considerations
- Intraoperatively, there should be a focus on maintaining stable hemodynamics and preventing seizures.8
- For children with known cardiac abnormalities, anesthetics should be administered cautiously to minimize myocardial depression and avoid drastic changes in systemic and pulmonary vascular resistance.6,7,9
- Invasive cardiac monitoring may be beneficial in some cases.7
- For patients with known pulmonary hypertension, intraoperative strategies to optimize pulmonary blood flow may include maintaining mild hypocarbia, high inspired oxygen, and low airway pressures.9
- Maintaining fluid balance and avoiding hypovolemia are essential in patients with preload-dependent heart defects.9
- Various anesthetic agents can be considered for the maintenance of anesthesia.
- For example, sevoflurane is known to have fewer cardiac depressant effects and may be a reasonable choice for a patient with congenital heart disease. However, sevoflurane has a relatively high epileptogenic risk.
- Isoflurane may decrease seizure occurrence, making it a better option for patients with recurrent seizures.6,8
- Bispectral index monitoring can be used to monitor seizure activity intraoperatively.8
- Maintaining normal EtCO2 levels can be used to prevent seizures caused by decreased cerebral blood flow.8
- For patients with impaired renal function, renally cleared medications should be used with caution.7,9
- Adequate renal blood flow can be maintained intraoperatively with a low-dose dopamine infusion to prevent kidney injury.7,9
- Limb contractures and flexion deformities of the extremities may pose challenges to vascular access as well as patient positioning.9
Recovery from Anesthesia8
- Patients with trisomy 13 and 18 frequently have obstructive or central sleep apnea, which can become apparent in the postoperative setting.
- Consider avoiding long-acting anesthetics, use opioids with caution, and closely monitor oxygen saturations in the postanesthesia recovery unit.
References
- Giersch A. Congenital cytogenetic abnormalities. In: Post T, ed. UpToDate; 2025. Link
- Balasundaram P, Avulakunta ID. Edwards syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. Link
- Cereda A, Carey JC. The trisomy 18 syndrome. Orphanet J Rare Dis. 2012;7:81. PubMed
- Lin H-Y, Lin S-P, Chen Y-J, et al. Clinical characteristics and survival of trisomy 18 in a medical center in Taipei, 1988–2004. Am J Med Genet A. 2006 140(9):945–51. PubMed
- Williams GM, Brady R. Patau Syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. Link
- Mifsud S, Bezzina M, Paris S. Anesthetic management of a patient with Edwards syndrome. Clin Case Rep. 2016;4(8):740-2. PubMed
- Amin M, Haroun L, Quraishi F, et al. Anaesthetic considerations in a 15-month-old with Patau's Syndrome: a case report and review of the literature. Anaesthesia Cases. 2017; 5: 18-22. Link
- Tsukamoto M, Hitosugi T, Esaki K, et al. The anesthetic management for a patient with Trisomy 13. Anesth Prog. 2017;64(3):162-4. PubMed
- Martlew RA, Sharples A. Anaesthesia in a child with Patau's syndrome. Anaesthesia. 1995;50(11):980-2. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.