Copy link
Transfusion Medicine and Pregnancy
Last updated: 09/23/2025
Key Points
- Approximately 1–2% of women require a blood transfusion during pregnancy or delivery. Neuraxial anesthesia may reduce the need for transfusion. Pregnant patients may not show signs of hypovolemia until the blood loss is greater than 25%.
- Goal-directed transfusion guided by labs or viscoelastic testing (e.g., rotational thromboelastometry [ROTEM]) improves outcomes by tailoring administration of packed red blood cells (pRBCs), fresh frozen plasma (FFP), fibrinogen, and platelets.
- RhoGAM prevents maternal alloimmunization by clearing fetal Rh⁺ RBCs before antibody formation. Failure to administer RhoGAM when indicated risks severe hemolytic disease and fetal/neonatal morbidity and mortality.
- Transfusion reactions (0.8% incidence postpartum) are usually non-severe but can include serious complications like transfusion-related acute lung injury (TRALI) and transfusion-associated circulatory overload (TACO) in massive hemorrhage.
Introduction
- Approximately 1–2% of women require a peripartum blood transfusion during pregnancy and delivery.1
- Key risk factors include antenatal anemia, cesarean delivery, Black race (mediated via anemia), placental issues (e.g., placenta previa, abruption), bleeding/coagulopathy disorders, operative vaginal delivery (forceps/vacuum), and multiple gestations.
- Labor neuraxial (epidural/spinal) analgesia is associated with reduced maternal transfusion odds, particularly in intrapartum cesareans.2
Pretransfusion Testing
Type & Screen (T&S)
- Determines ABO blood type, Rh status, and screens for unexpected antibodies (e.g., anti-D, anti-Kell).
- Routine pre-transfusion testing when transfusion is not yet certain but possible (e.g., in uncomplicated labor, C-section preparation).
- This test is valid for only 72 hours in pregnancy due to the risk of alloimmunization.
- If the “screen” for antibodies is positive, the next step is antibody identification testing.
- Timing
- A T&S takes approximately 30 minutes to 1 hour when no antibodies are identified.
- If antibodies are identified, the time to complete the test varies depending on the rarity of the antibodies in question. For common antibodies, this typically spans a few hours, while for rare types, it can take several days.
- If a second confirmatory lab is required by the blood bank to confirm the patient’s blood type, an ABO Group/Rh test can typically be performed in 15 minutes.
Type & Crossmatch (T&C)
- Purpose: Reserves specific units of RBCs after compatibility testing (crossmatching).
- Used when transfusion is likely or imminent (e.g., bleeding, emergency C-section, massive obstetric hemorrhage).
- Includes T&S, then proceeds to match actual donor units with the patient’s serum.
- Timing
- Timing again is dependent upon whether or not antibodies are identified.
- If there are no antibodies, a T&C takes approximately 2 hours in total.
- If there are antibodies, the timing depends on the rarity of the antibody as well as the selection at the hospital’s blood bank. This can be on the order of hours for common antibodies to days for rare blood types to find an appropriate match.
Transfusion Strategies
- Maternal blood volume expands in pregnancy. In pregnant patients, tachycardia and hypotension usually do not present until there is a substantial blood loss. Signs of hypovolemia usually represent a loss of 25% of the woman’s total blood volume (approximately 1,500 mL).
- Fixed-ratio transfusion may not be optimal in pregnant patients who have baseline changes in coagulation factors. Because of this, goal-directed transfusion is recommended.3
- Transfusion therapy guided by viscoelastic monitoring rather than a fixed ratio reduces the use of plasma and platelets without increasing blood loss or coagulation problems.4
pRBC
- The goal is a hemoglobin (Hb) of greater than 7 or 8 g/dL. In the absence of ongoing bleeding, 1 unit of pRBC should increase the Hb by 1 g/dL.
- The risks of pRBC administration, such as transfusion reactions and alloimmunization, are detailed below.
FFP
- During pregnancy, plasma levels of clotting factors are significantly higher. Therefore, FFP is probably of limited usefulness for the routine treatment of postpartum hemorrhage. It may still be necessary in cases of massive transfusion or disseminated intravascular coagulation.
- Some blood centers may accept FFP from female donors who have never been pregnant. That being said, many places preferentially use a male donor population. The rationale is that the female donor may’ve had a pregnancy she did not know about and subsequently have human leukocyte antigen antibodies present in plasma that increase the risk of TRALI.
- The average international normalized ratio (INR) of FFP is 1.1. Therefore, by giving FFP, an elevated INR can be normalized.
- The goal of FFP administration is an INR value less than 1.5 times the upper limit of normal.
Fibrinogen
- The consumption of fibrinogen plays a key role in obstetric hemorrhage.
- One study found that a fibrinogen concentration of less than 200 mg/dL at the time of diagnosis had a 100% positive predictive value for severe hemorrhage. On the other hand, a fibrinogen concentration of greater than 400 mg/dL had a 79% negative predictive value.5
- Fibrinogen levels can be repleted with either cryoprecipitate or fibrinogen concentrate
- Cryoprecipitate: A 10-unit pool should increase the fibrinogen level by 50-100 mg/dL
- Fibrinogen Concentrate: Initial dose for a 70 kg patient is 2-4 g
- The goal is either fibrinogen > 200, or on ROTEM, a FIBTEM A5 of > 10.4
Platelets
- Transfusion is indicated when the platelet level is less than 50,000. Thrombocytopenia to less than 50,000 in obstetric hemorrhage is rare unless there is an estimated blood loss greater than 5L or a consumptive coagulopathy is present.
- One unit of platelets increases the platelet count by 5,000 to 10,000.
Adjunct Agents in Hemorrhage
- Tranexamic acid (TXA) is often given empirically in the event of hemorrhage. If the ROTEM shows an EXTEM Maximum Lysis of greater than 15%, this could indicate TXA is needed.
- The dose is 1 g IV over 10 minutes, and it can be repeated after 30 minutes if there is ongoing bleeding.
- Recombinant factor VIIa has been used as a last-resort (“rescue”) therapy in massive obstetric hemorrhage refractory to conventional treatment. There is a risk of thrombosis, so we recommend a small starting dose of 5-15 mcg/kg.
Transfusion Endpoints
- The decision to stop transfusion is multifaceted and relies on clinical judgement. Some suggested endpoints include:
- Cessation of bleeding
- Hemodynamic stability
- Urine output 0.5 mL to 1 mL/kg/hr
- Decreasing serum lactate
- Stabilization of lab measures of coagulation
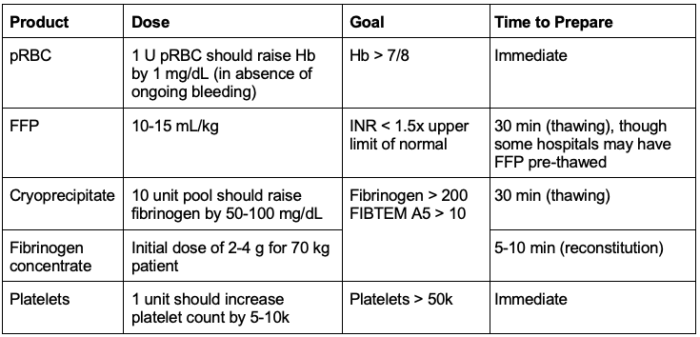
Table 1. Goal-directed transfusion for labor and delivery. Abbreviation: pRBC, packed red blood cells; FFP, fresh frozen plasma; Hb, hemoglobin; INR, international normalized ratio
Alloimmunization
- During a normal pregnancy, fetal cells transfer into the mother’s bloodstream. Alloimmunization happens when the mother develops IgG antibodies against fetal RBC antigens inherited from the father that are not her own. This poses a risk for hemolytic disease of the newborn (HDN) in future pregnancies.
- Most clinically important antibodies target Rh (Rhesus), Kell, Duffy, and Kidd antigens.
- In HDN, maternal anti-D antibodies attack fetal RBCs, causing anemia, hyperbilirubinemia, hydrops fetalis, and fetal death.
- Rh(D) immunoglobulin (RhoGAM) should be administered at 28 weeks’ gestation to all Rh(-) patients and again within 72 hours postpartum to Rh(-) patients who deliver Rh‑positive infants to prevent alloimmunization.
- RhoGAM contains anti-D immunoglobulin (IgG antibodies) that bind to any Rh⁺ fetal RBCs circulating in the mother’s bloodstream. By this mechanism, RhoGAM masks the Rh(D) antigen and facilitates its removal by the mother’s spleen before her immune system can recognize and react to it.
- RhoGAM should be given after any event that could cause fetal blood to enter maternal circulation (trauma, amniocentesis, spontaneous abortion).
- If RhoGAM is not given, the patient is at risk of alloimmunization and HDN in subsequent pregnancies.
- A large multinational study investigating all cases of HDN because of alloimmunization found that 83% of cases were due to previous pregnancy and 3% due to a previous transfusion (14% etiology undetermined).6
- Overall, alloimmunization occurs in ~1–2% of pregnancies in high-income countries with routine RhoGAM prophylaxis.
Rare Blood Types
- For patients with rare blood types or multiple antibodies, it can be challenging to obtain compatible units of pRBCs. It is important to coordinate with the blood bank in antenatal planning to ensure there is a plan for blood to be available.
- In some cases, autologous blood donation or frozen rare blood units may be necessary to ensure availability during delivery.
- Rh-null
- Patients who are Rh-null have a complete absence of all Rh antigens on their RBCs. This means they can only receive Rh-null blood transfusions, complicating transfusion management.
- In pregnancy, Rh-null patients are at risk of alloimmunization.
- Some rare blood types are more prevalent in ethnically isolated populations, including Pacific Islanders, Melanesians, and Micronesians. These rare antigens may not be on standard Western screens.
Cell Salvage in Labor & Delivery
- Cell salvage allows for the collection and reinfusion of autologous blood.
- Indications for cell salvage include:
- Anticipated blood loss greater than 500 mL or more than 10% estimated blood volume
- Low starting hematocrit
- Patients with increased risk factors for bleeding
- Patients with multiple antibodies
- Patients who refuse donor products and blood loss is anticipated
- There are potential risks of cell salvage in labor and delivery, including (1) alloimmunization and (2) amniotic fluid embolism (AFE).
Alloimmunization
- Fetal RBCs cannot be differentiated from maternal RBCs in the cell salvage process and are present in the returned blood.
- In a series of 436 patients who received salvaged blood, there was only 1 case of alloimmunization in a subsequent pregnancy.7
AFE
- To avoid contamination of the blood with amniotic fluid, it is common practice to use separate suction sources for blood and amniotic fluid between the uterine incision and the delivery of the placenta.
- Additionally, modern cell salvage systems remove tissue factor (a possible cause for AFE) from collected blood during cesarean delivery. Cell salvage with the addition of leukocyte depletion filters reduces fetal squamous cell levels to a concentration equivalent to maternal venous blood.8
- Furthermore, there have been no confirmed cases of AFE from salvaged blood, so the risk remains theoretical. Therefore, the use of intrapartum cell salvage is likely safe.
Transfusion Reactions
- Among women who received pRBC, plasma, or platelet transfusions after delivery, 0.8% experienced a transfusion reaction. This is about twice the rate seen in non-pregnant women (0.4%).9
- Transfusion reactions can be categorized into acute vs. delayed and immune vs. non-immune mediated.
- The majority of transfusion reactions seen are non-severe. In heavily transfused patients (e.g. in the event of massive hemorrhage), the risks of TRALI and TACO greatly increase.
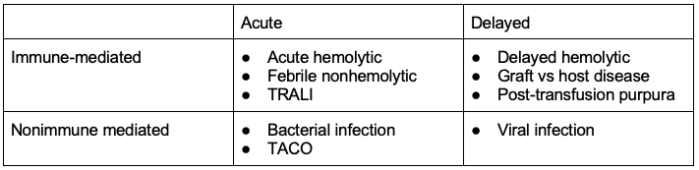
Table 2. Transfusion reactions. Abbreviations: TACO, transfusion-associated circulatory overload; TRALI, transfusion-related acute lung injury
Ethics of Blood Refusal
- Patients may refuse blood for a variety of reasons, a common reason being a religious objection amongst Jehovah’s Witnesses.
- There are many blood products available, and patients from the same religious group can vary significantly in the products they accept. Therefore, the clinician should go through a transfusion consent sheet with the patient and clearly document which products are and are not acceptable.
- If a patient has previously expressed religious or cultural objections to transfusion, it must be withheld, even at risk of death, unless there is clear, contrary evidence.
- If a previously competent patient is now incapacitated but had clearly refused a blood transfusion, the presumption is to honor that refusal, even in life-threatening situations.
- Patients always retain the right to change their minds about accepting treatment.
References
- Klapholz H. Blood transfusion in contemporary obstetric practice. Obstet Gynecol. 1990;75(6):940-3. PubMed
- Guglielminotti J, Landau R, Daw J, Friedman AM, Li G. Association of Labor Neuraxial Analgesia with Maternal Blood Transfusion. Anesthesiology. 2023;139(6):734-45. PubMed
- Waters JH, Bonnet MP. When and how should I transfuse during obstetric hemorrhage? Int J Obstet Anesth. 2021;46:102973. PubMed
- Mallaiah S, Barclay P, Harrod I, Chevannes C, Bhalla A. Introduction of an algorithm for ROTEM-guided fibrinogen concentrate administration in major obstetric haemorrhage Anaesthesia. 2015;70(2):166-175. PubMed
- Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost. 2007;5(2):266-273. PubMed
- Delaney M, Wikman A, van de Watering L, et al. Blood Group Antigen Matching Influence on Gestational Outcomes (AMIGO) study. Transfusion. 2017;57(3):525-32. PubMed
- Leeson C, Jones M, Odendaal J, Choksey F, Quenby S. Routine use of cell salvage during cesarean section: A practice evaluation. Acta Obstet Gynecol Scand. 2024;103(3):498-504. PubMed
- Waters JH, Biscotti C, Potter PS, Phillipson E. Amniotic fluid removal during cell salvage in the cesarean section patient. Anesthesiology. 2000;92(6):1531-6. PubMed
- Thurn L, Wikmanm A, Westgren M, Lindqvist P; Incidence and risk factors of transfusion reactions in postpartum blood transfusions. Blood Adv. 2019; 3 (15): 2298–2306. PubMed
Other References
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.