Copy link
Target Controlled Systems and Closed Loop Systems
Last updated: 01/26/2024
Key Points
- Target-controlled infusion (TCI) uses population-level models to achieve predictable target blood drug concentrations.
- Closed-loop systems use feedback from physiologic monitoring data, such as processed electroencephalography, hemodynamics, and ventilation, to deliver and titrate patient-specific medication administration in real-time.
- The advantages of closed-loop systems include greater precision, cost efficacy, reduced cognitive load, and the potential for human errors.
Introduction
- Proper drug administration is paramount to patient safety. The five rights of medication administration include the right patient, right drug, right dose, right time, and right route. This is particularly relevant in the perioperative setting, where a single provider may have to rapidly prescribe, dispense, and administer a wide range of medications without supervision.
- Furthermore, manual administration of medications, particularly in an urgent or emergent situation, invites the potential for human error and adverse outcomes. The advent of automated medication delivery devices has introduced an alternative way to administer medications in a fast, simple, and precise manner that can increase patient safety and decrease the work burden on the provider.
- Devices utilizing model-based administration are able to perform computer-controlled measurements and dispense a stable, consistent, and reliable medication concentration to patients. An important consideration is whether the provider wishes to target a blood-drug concentration based on population-level models and drug-dosing weight or rather maintain a target therapeutic effect mediated by the patient’s physiological responses. TCI and closed-loop systems are two methods by which these management goals can be achieved, respectively.
Target-Controlled Infusions
- TCI is a system where computer simulation of a known infusion scheme directs the selection of specific pharmacokinetic parameters. A computer-compatible infusion pump then uses these specific parameters to deliver the appropriate concentration of various medications.1
- Repeated doses or continuous infusions of many medications without adequate clearance can lead to accumulation and even toxicity. This is of particular importance when using total intravenous anesthetics (TIVA). TCI systems for the infusion of sedatives, opioids, and other anesthetics have been developed to achieve specific targeted blood concentrations.
- The process of TCI operation includes the following steps.2
- A computer pump determines the best model for a selected drug by incorporating patient metrics (i.e., weight, height, age, sex) and pharmacokinetic properties of the drug being dispensed to then calculate the amount of drug that will be needed to reach a target concentration. Note that model selection may affect the metrics that can be included.
- A target drug concentration is entered into a computer by the clinician.
- An infusion pump connected to the computer delivers the calculated amount of drug to the patient.
- The computer provides a constant measure of tissue concentration as the infusion is delivered and then compares this concentration to the selected model for the drug being dispensed. The computer can then adjust the infusion rate accordingly to maintain a stable concentration. The result is a more precise control over drug delivery and effect, especially in situations where rapid titration is necessary.
- TCI systems are commonly based on the three-compartment model, which models the pharmacokinetic properties of intravenously administered drugs as they move through three key phases in the body: distribution, elimination, and tissue release.3
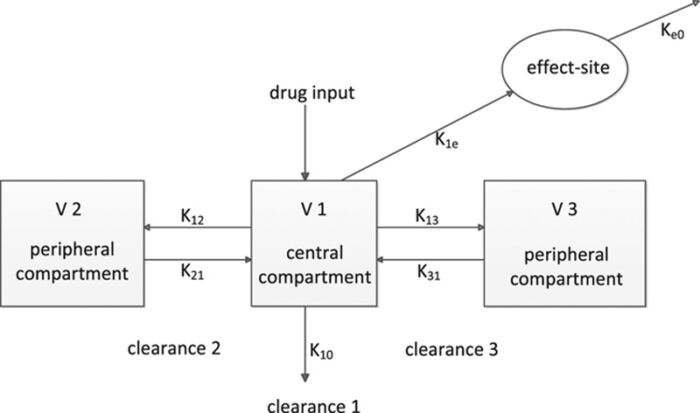
Figure 1. The 3-compartment model with 3 volumes of distribution (V1, V2, and V3) and various k values representing the rate and direction of movement of the drug among the compartments (k12, k21, k13, and k31) or toward the outside (k10). The drug is administered into a central compartment, from which it is eliminated. The drug is rapidly and slowly distributed in a second and third compartment, respectively. The delay in equilibration between the plasma and effect-site concentration is mathematically described by ke0, the effect-site equilibration rate constant. Used with permission from Struys MMRF, et al. The history of target-controlled infusion. Anesth Analg. 2016.2
- TCI developed slowly over three decades with extensive research and experimentation and is now considered a mature technology leading the movement towards computer-assisted anesthesia. However, it has still not received regulatory approval in the United States outside the use in research studies.3
- Close to 30 years of global clinical use has yielded only seven reports of pump malfunction or drug-swap error, none of which had clinically significant outcomes.4 Additionally, there have been no reported errors or safety issues related to the pharmacokinetically based infusion algorithm TCI systems use to operate. While it is possible that safety concerns could be underrepresented by the lack of reports, thus far, TCI systems do not appear to pose any significant safety issues.
- Disadvantages of using TCI systems include5,6
- patient variability in the pharmacokinetic and pharmacodynamic parameters used in models to calculate the effect site drug concentration. For example, obesity is associated with higher than anticipated plasma concentrations of propofol.
- Most TCI models do not compensate for patient comorbidities.
- Most TCI models do not compensate for drug-drug interactions when multiple medications are administered.
Closed-Loop Systems
- Closed-loop systems analyze input signals and modulate the output in response to parameters defined in the input signal (Figure 2).7-9 An example of a closed-loop system is an insulin pump and continuous glucose monitor.
- An input signal (blood glucose level) is received.
- The signal is interpreted in the context of parameters set on the input (ideal blood glucose range for the patient).
- The output responds accordingly (insulin release from the pump).
- By definition, closed-loop systems function independently without human intervention. Human-in-the-loop systems are similar but allow for human intervention to alter the system’s course or account for other variables when necessary.
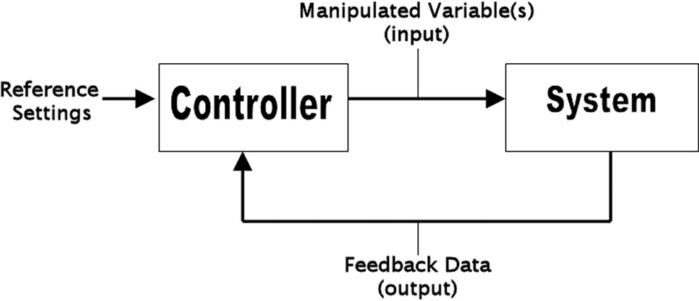
Figure 2. Closed-loop system. The controller is given reference settings, which are then compared with the feedback data (system output). The difference between the two is the error, which is used to generate a new system input. Used with permission from Rinehart J, et al. Review article: closed-loop systems in anesthesia: is there a potential for closed-loop fluid management and hemodynamic optimization? Anesth Analg. 2012.7
- Closed-loop systems are able to exert control over a wide range of variables, including hypnosis, analgesia, neuromuscular blockade, temperature, metabolic status, ventilation, and hemodynamics, all through the use of a feedback loop8 (Figure 3).
- Positive feedback: the feedback signal adds to the actual input to amplify the effective input to the system.
- Negative feedback: the feedback signal is subtracted from the actual input, decreasing the overall input to the system.
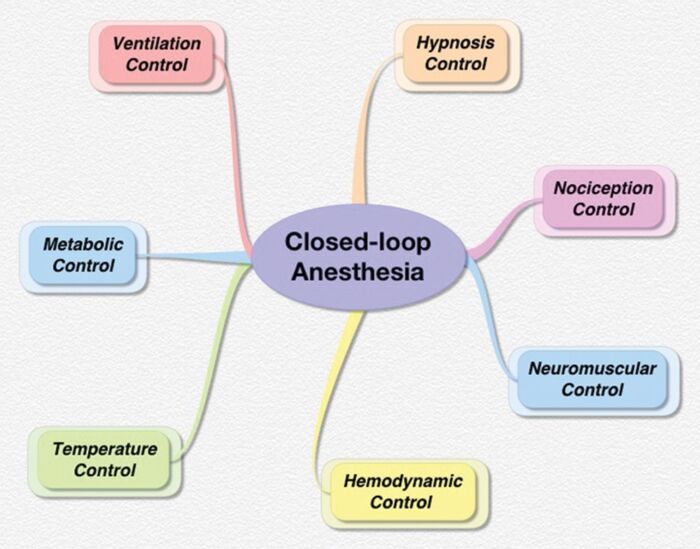
Figure 3. Potential variables that can be controlled by closed-loop systems. Used with permission from Dumont GA, et al. Closed-loop control of anesthesia: a primer for anesthesiologists. Anesth Analg. 2013.7
- A key distinction between open- and closed-loop systems is that closed-loop systems are not dependent on models to perform properly. This allows them to operate very accurately under conditions where there is significant uncertainty or the potential for disturbances to the system. For this reason, the development of their use in the perioperative setting is of great interest.7 Draeger has introduced the Zeus® Infinity® with agent control, and a three-drug anesthesia physiologic closed-loop control technology has been approved in China.
- Major advantages of closed-loop systems to manual adjustment include the following.7-9
- Intravenous anesthetic depth is controlled with greater precision, specific to the individual patient, without reliance on population-level models.
- A meta-analysis of randomized control trials concluded that bispectral index monitoring of a propofol closed-loop target-controlled infusion decreased the amount of propofol administered, the incidence of abnormal blood pressure, and postoperative cognitive dysfunction.5
- Reduced potential for human error, especially in multivariable settings with a high degree of uncertainty
- Reduced cognitive load and repetitive tasks associated with the manual adjustment of infusion devices
- Greater cost efficacy
- Closed-loop systems do present potential safety concerns; “runaway” behavior leading to significant instability can occur if feedback loops are not well constructed, or if the clinician interferes with the feedback loop too regularly. Of particular concern is the possibility that a system is no longer able to achieve its goal and must hand over full control to the clinician, who must be aware of the state of the system in order to maintain stability.
- Closed-loop systems are not a replacement for the anesthesiologist and the real-time decision-making of a clinical team. The ideal use of these systems is to help eliminate unimportant variability (i.e., frequent infusion-rate changes) as well as undesired variability (i.e., hemodynamic) thus allowing the anesthesia provider more freedom to perform higher-level tasks.8,12
- A meta-analysis of the clinical performance and safety of closed-loop systems concluded that closed-loop automated systems provided greater stability of controlled variables and reduced clinician workload at no cost to patient safety. Significant decreases in overshooting and undershooting of anesthetic drug delivery to diabetic and mechanically ventilated patients were observed.12
References
- Guarracino F, Lapollla F, Cariello C, et al. Target controlled infusion: TCI. Minerva Anestesiol. 2005; 71(6):335-7. PubMed
- Struys MMRF, De Smet T, Glen JB, et al. The history of target-controlled infusion. Anesth Analg. 2016;122(1):56-69. PubMed
- Absalom AR, Glen JI, Zwart GJ, et al. Target-controlled infusion: A mature technology. Anesth Analg. 2016;122(1):70-8. PubMed
- Schnider TW, Minto CF, Struys MM, Absalom AR. The safety of target-controlled infusions. Anesth Analg. 2016;122(1):79-85. PubMed
- Engbers FHM, Dahan A. Anomalies in target-controlled infusion: an analysis after 20 years of clinical use. Anaesthesia. 2018;73(5): 619-30. PubMed
- Ingrande J, Lemmens HJ. Target-controlled infusion: not a one-sized-fits-all answer to drug administration. Anesth Analg. 2018; 127(4): 813-4. PubMed
- Rinehart J, Liu N, Alexander B, et al. Review article: closed-loop systems in anesthesia: is there a potential for closed-loop fluid management and hemodynamic optimization? Anesth Analg. 2012;114(1):130-43. PubMed
- Dumont GA, Ansermino JM. Closed-loop control of anesthesia: a primer for anesthesiologists. Anesth Analg. 2013;117(5):1130-8. PubMed
- Miller TE, Gan TJ. Closed-loop systems in anesthesia: Reality or fantasy? Anesth Analg. 2013;117(5):1039-41. PubMed
- Joosten A, Rinehart J, Van der Linden P, et al. Computer-assisted individualized hemodynamic management reduces intraoperative hypotension in intermediate- and high-risk surgery: A randomized controlled trial. Anesthesiology. 2021;135(2):258-72. PubMed
- Wang D, Song Z, Zhang C, et al. Bispectral index monitoring of the clinical effects of propofol closed-loop target-controlled infusion: Systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2021;100(4):e23930. PubMed
- Brogi E, Cyr S, Kazan R, et al. Clinical performance and safety of closed-loop systems: A systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2017;124(2):446-55. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.