Copy link
Reversal of Neuromuscular Blockade: Sugammadex
Last updated: 04/06/2023
Key Points
- Sugammadex is the first selective relaxant binding agent designed to bind rocuronium.
- It has a novel mechanism of action, encapsulating and thereby inactivating aminosteroidal nondepolarizing neuromuscular blocking agents.
- Sugammadex can reverse all levels of neuromuscular blockade produced by aminosteroidal nondepolarizing neuromuscular blocking agents.
- Sugammadex does not have parasympathetic side effects and does not require the coadministration of an antimuscarinic agent.
Introduction
- Recovery from the effects of nondepolarizing neuromuscular blocking agents (NMBAs) may occur spontaneously via redistribution, metabolism, and excretion. Recovery from NMBAs can also be facilitated by administering an antagonist or “reversal agent.”
- Antagonism of NMBAs in the United States has historically been accomplished with anticholinesterase agents such as neostigmine until the introduction of a novel antagonist, sugammadex.
- Sugammadex was introduced into anesthesia practice internationally in 2008. The United States Food and Drug Administration (FDA) approved its use in adults in 2015 and pediatric patients in 2021.
Structure of Sugammadex
- Sugammadex is a modified gamma-cyclodextrin with eight additional side chains containing negatively charged carboxyl groups, creating a molecule with a deep hydrophobic cavity and enhanced electrostatic binding for rocuronium (Figure 1).1
- While developed specifically for rocuronium antagonism, sugammadex binds other aminosteroidal NMBAs with an affinity for rocuronium > vecuronium > pancuronium.
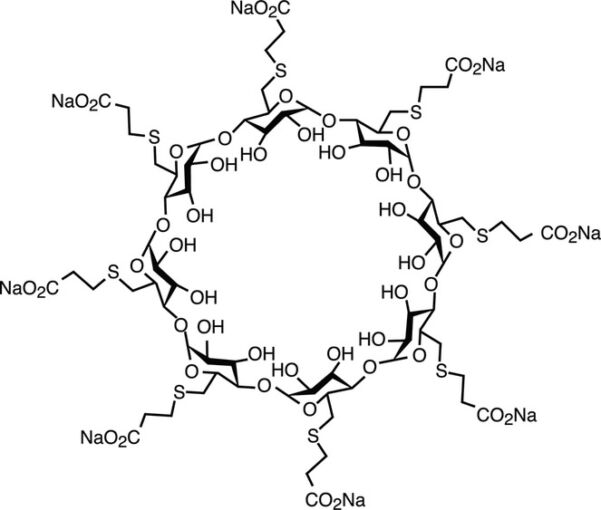
Figure 1. Sugammadex is a synthetic gamma-cyclodextrin that has been modified to encapsulate aminosteroidal nondepolarizing NMBAs. Adapted from Naguib M. Anesth Analg. 2007.1
Mechanism of Action
- Sugammadex works by encapsulating aminosteroidal nondepolarizing NMBAs (Figure 2).1 Binding occurs at a 1:1 molar ratio in a very tight relationship that is essentially nonreversible.
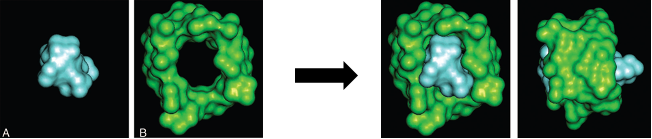
Figure 2. Encapsulation of rocuronium by sugammadex. Radiograph crystal structure of rocuronium (A), sugammadex (B) and a rocuronium/sugammadex complex (C and D). Used with permission from Naguib M. Anesth Analg. 2007.1
- Encapsulation of NMBAs in the plasma prevents their diffusion into the neuromuscular junction (NMJ) and creates a gradient that favors the movement of NMBA molecules away from the NMJ and into the plasma, where they are also bound by free sugammadex molecules and inactivated (Figure 3).
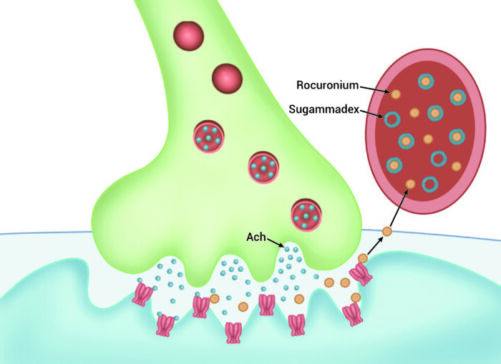
Figure 3. Encapsulation of free rocuronium in the plasma creates a gradient that favors the movement of unbound rocuronium from the NMJ into the plasma for further binding and inactivation by sugammadex.
- This mechanism of action allows antagonism of profound levels of neuromuscular blockade and immediate reversal of NMBA after their administration in “can’t intubate/can’t ventilate” situations.
- Sugammadex is unable to reverse blockade with benzylisoquinolinium NBMAs.
- Re-establishing neuromuscular blockade following sugammadex administration can be accomplished with benzylisoquinolinium NMBAs.
Sugammadex vs Neostigmine
- Sugammadex is recommended over neostigmine for reversal of deep, moderate, and shallow levels of blockade when antagonizing rocuronium- or vecuronium-induced neuromuscular blockade.2
- Sugammadex antagonizes all depths of blockade more rapidly and with a better safety profile than neostigmine.3,4
- The use of sugammadex can improve rates of residual neuromuscular blockade.3,5
- The impact of sugammadex in preventing postoperative pulmonary complications has been reported but remains debated.6,7
Adverse Effects
- Its mechanism of action compared to anticholinesterases agents gives sugammadex the advantage of absent muscarinic side effects, eliminating the need for the administration of antimuscarinic agents in conjunction with sugammadex.
- Potential adverse effects include:
- Bradycardia – likely dose-dependent, but unknown mechanism. Treat with vasopressors or anticholinergics.
- Anaphylaxis – Reported hypersensitivity reactions have been seen within the first five minutes of administration.
- Heme disturbances – initial concerns for prolongation of prothrombin time and partial thromboplastin time with the 16mg/kg dose of sugammadex, but this was found to be a lab artifact.
Special Considerations
- Sugammadex and sugammadex/NMBA complex is renally excreted, so its use in patients with renal failure is not recommended.
- Possible interference with steroidal (hormonal) methods of birth control. All women of childbearing age who have received sugammadex should be counseled to use alternative methods of birth control for 1 week after sugammadex administration.8
- The use of sugammadex is not recommended in early pregnancy because it binds to and encapsulates progesterone which is critical for the maintenance of pregnancy.8
- For patients at term or near-term pregnancy requiring general anesthesia for cesarean delivery, sugammadex use appears to be safe but should be used judiciously since its effects on lactation are not known.8
- In certain obstetric patients, the benefits of sugammadex use may outweigh the theoretical or unknown risks to fertility, pregnancy, or lactation.8
- Patients with an unanticipated difficult airway (cannot intubate, cannot ventilate)
- Patients in whom the administration of neostigmine has reached a ceiling effect but remain at an elevated risk for inadequate reversal (e.g., high-dose magnesium therapy, myasthenia gravis, etc.)
- FDA dosing indications for pediatric patients do not include children younger than 2 years of age, and the dosing indication for the immediate reversal of NMBAs with a 16mg/kg dose is not available for any pediatric age group.9
Dosing and Timing of Administration
- Despite its greater margin of safety compared to neostigmine, the use of a peripheral nerve stimulator (PNS), or ideally quantitative monitoring, is still necessary to determine the dosing and timing of administration for sugammadex
- Quantitative monitoring remains the only means to determine whether full recovery to a train-of-four ratio ≥0.9 has been achieved.
- Residual neuromuscular blockade due to improper dosing of sugammadex has been reported, especially in the absence of monitoring.10
- FDA dosing recommendations for adults and children are listed in Figure 4.
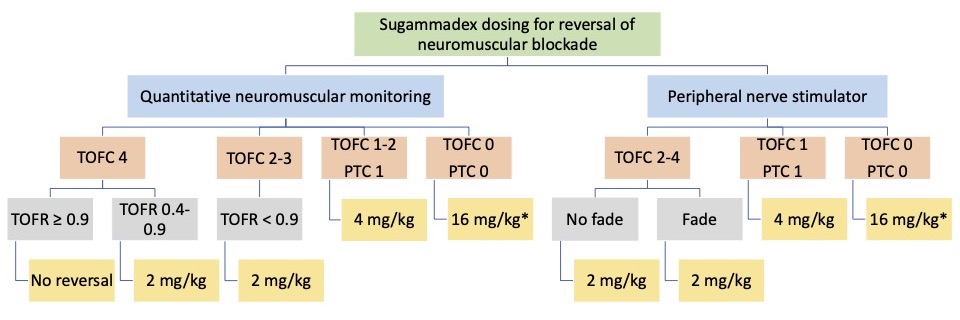
Figure 4. Sugammadex dosing according to the depth of neuromuscular blockade measured with a qualitative peripheral nerve stimulator (PNS) or quantitative neuromuscular monitoring (QNM). TOFC=train-of-four count; TOFR=train-of-four ratio; PTC = post-tetanic count. *Dosing indication with a 16mg/kg dose is not available for children. Additionally, no dosing indications exist to reverse any level of blockade in children less than 2 years of age.
References
- Naquib M. Sugammadex: another milestone in clinical neuromuscular pharmacology. Anesth Analg. 2007;104(3): 575-81. PubMed
- Thilen SR, Weigel WA, Todd MM, et al. 2023 American Society of Anesthesiologists practice guidelines for monitoring and antagonism of neuromuscular blockade: A report by the American Society of Anesthesiologists Task Force on neuromuscular lockade. Anesthesiology. 2023;138(1):13-41. PubMed
- Hristovska AM, Duch P, Allingstrup M, et al. The comparative efficacy and safety of sugammadex and neostigmine in reversing neuromuscular blockade in adults. A Cochrane systematic review with meta-analysis and trial sequential analysis. Anaesthesia. 2018;73(5):631-41. PubMed
- Gaver RS, Brenn BR, Gartley A, et al. Retrospective analysis of the safety and efficacy of sugammadex versus neostigmine for the reversal of neuromuscular blockade in children. Anesth Analg. 2019;129(4):1124-9. PubMed
- Togioka BM, Yanez D, Aziz MF, et al. Randomised controlled trial of sugammadex or neostigmine for reversal of neuromuscular block on the incidence of pulmonary complications in older adults undergoing prolonged surgery. Br J Anaesth. 2020;124(5):553-61. PubMed
- Kheterpal S, Vaughn MT, Dubovoy TZ, et al. Sugammadex versus Neostigmine for Reversal of Neuromuscular Blockade and Postoperative Pulmonary Complications (STRONGER): A Multicenter Matched Cohort Analysis. Anesthesiology. 2020;132(6):1371-81. PubMed
- Kirmeier E, Eriksson LI, Lewald H, et al. Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): a multicentre, prospective observational study. Lancet Respir Med. 2019;7(2):129-140. PubMed
- Willett, Butwick, Togioka, et al. Statement on sugammadex during pregnancy and lactation. Society for Obstetric Anesthesia and Perinatology. 2019. Accessed January 2023. Link
- Voss T, Wang A, DeAngelis M, et al. Sugammadex for reversal of neuromuscular blockade in pediatric patients: Results from a phase IV randomized study. Paediatr Anaesth. 2022;32(3):436-45. PubMed
- Kotake Y, Ochiai R, Suzuki T, et al. Reversal with sugammadex in the absence of monitoring did not preclude residual neuromuscular block. Anesth Analg. 2013;117(2):345-51. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.