Copy link
Renal Anatomy and Physiology
Last updated: 05/06/2025
Key Points
- The kidneys maintain fluid and electrolyte balance, filter metabolic waste, and produce hormones that regulate blood pressure, red blood cell production, and bone metabolism.
- The kidneys receive about 20% of the cardiac output. Renal blood flow and glomerular filtration rate (GFR) are autoregulated through myogenic and tubuloglomerular mechanisms, ensuring stable function within a pressure range of 80–180 mm Hg.
- Anesthesia and surgical stress can reduce GFR and urine output, though the effects are usually mild and transient. Methoxyflurane is the only proven anesthetic agent that is directly nephrotoxic.
- Compound A, a breakdown product of sevoflurane, causes acute kidney injury in laboratory animals, especially at low fresh gas flow rates. However, no clinical study in humans has detected significant kidney injury.
- Compound A, a breakdown product of sevoflurane, causes acute kidney injury in laboratory animals, especially at low fresh gas flow rates. However, no clinical study in humans has detected significant kidney injury.
Introduction
- The kidneys play a vital role in sustaining extracellular fluid balance, which is vital for normal cellular activity. This is achieved by filtering metabolic waste products (i.e., urea, creatinine, uric acid) and by adjusting water and electrolyte excretion based on the body’s intake and internal production. Solutes such as sodium, potassium, and hydrogen are regulated independently through changes in tubular absorption and secretion.1
- In addition to their excretory function, the kidneys act as endocrine organs by producing hormones that influence blood pressure regulation (renin, prostaglandins, bradykinin), red blood cell synthesis (erythropoietin), and bone mineral metabolism (calcitriol).1
Renal Anatomy
- The kidneys are paired organs about the size of your fist and shaped like beans.2 They are retroperitoneal organs that lie between the parietal peritoneum and the posterior abdominal wall. The right kidney lies slightly lower than the left kidney.2
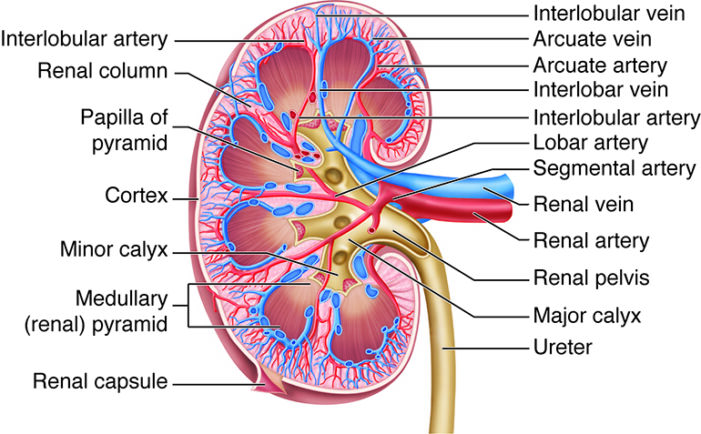
- The kidneys have a concave medial border, and there is a depression near the middle of the concave medial border, called the renal hilum, where the renal artery enters the kidney and the renal vein and ureter leave the kidney (Figure 1).
- A renal capsule on the external surface preserves the form of the kidney.
- The internal anatomy of the kidneys includes the renal cortex and medulla. The renal cortex is the outer layer of the kidney, and it also extends as renal columns between the renal pyramids.2
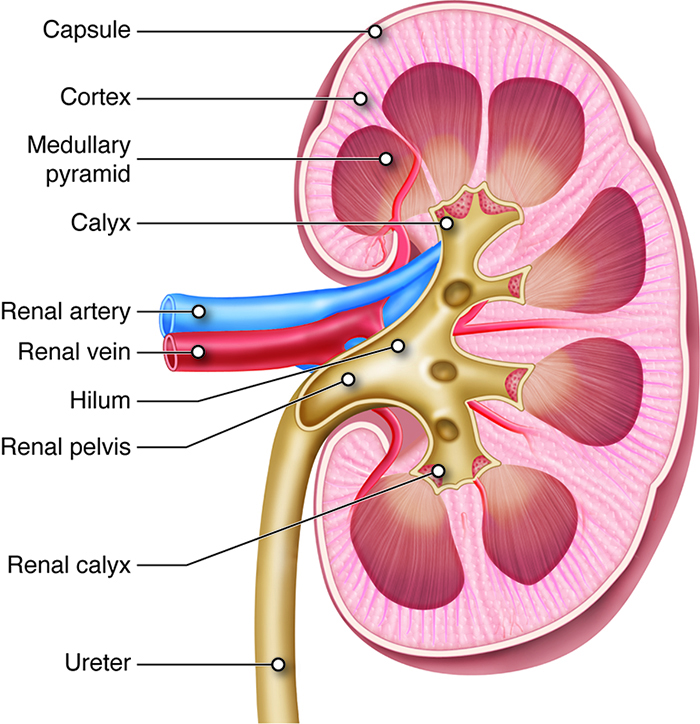
Figure 1. Cross section of a kidney. Source: Anatomy and Physiology by SBCCOE. CC BY NC SA 4.0. Link
- The renal pyramids make up the renal medulla, and their primary role is to maintain the proper balance of salt and water in the blood. The renal papillae are located at the apex of the renal pyramids and face the renal hilum.2
- A renal lobe consists of one renal pyramid and its surrounding cortex.
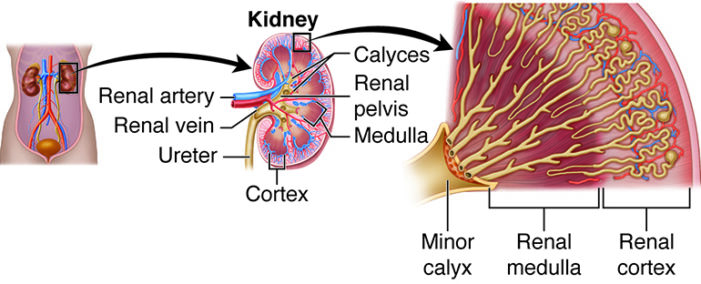
Figure 2. Internal structure of the kidney. Source: Anatomy and Physiology by SBCCOE. CC BY NC SA 4.0. Link
Renal Blood Supply
- The kidneys are the only organs for which oxygen consumption is determined by blood flow; the reverse is true in other organs.3
- Approximately 20-25% of the cardiac output (about 1 liter per minute) is delivered to the kidneys. Renal blood flow is governed by the pressure difference between the renal artery and vein and is inversely influenced by vascular resistance. Due to the parallel arrangement of renal vessels, total resistance is minimized, facilitating efficient perfusion.3
- Renal blood flow begins at the renal artery and is directed through progressively smaller vessels—segmental, interlobar, arcuate, and interlobular arteries. From the interlobular arteries, afferent arterioles transport blood to the glomeruli, where filtration occurs (Figure 3). Efferent arterioles then carry blood away, forming peritubular capillaries in the cortex or descending into the medulla to form the vasa recta, which supply the medullary tissue.3
- Veins follow the same courses as arteries, but in reverse.2
- Higher perfusion in the cortex helps maintain an interstitial environment similar to plasma, whereas reduced medullary flow supports the osmotic gradient required for urine concentration and water reabsorption.3
- Innervation of the kidneys is supplied from an outgrowth of the celiac plexus, called the renal plexus. This complex of autonomic nerve fibers and ganglia is primarily supplied by sympathetic vasomotor fibers.
Primary Functional Components of the Kidney
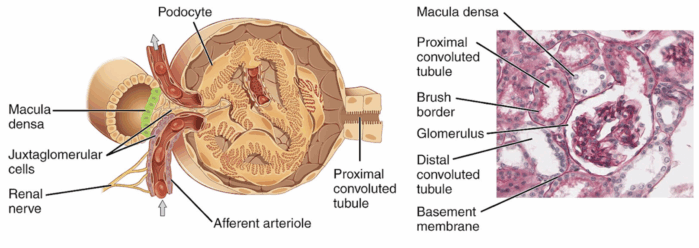
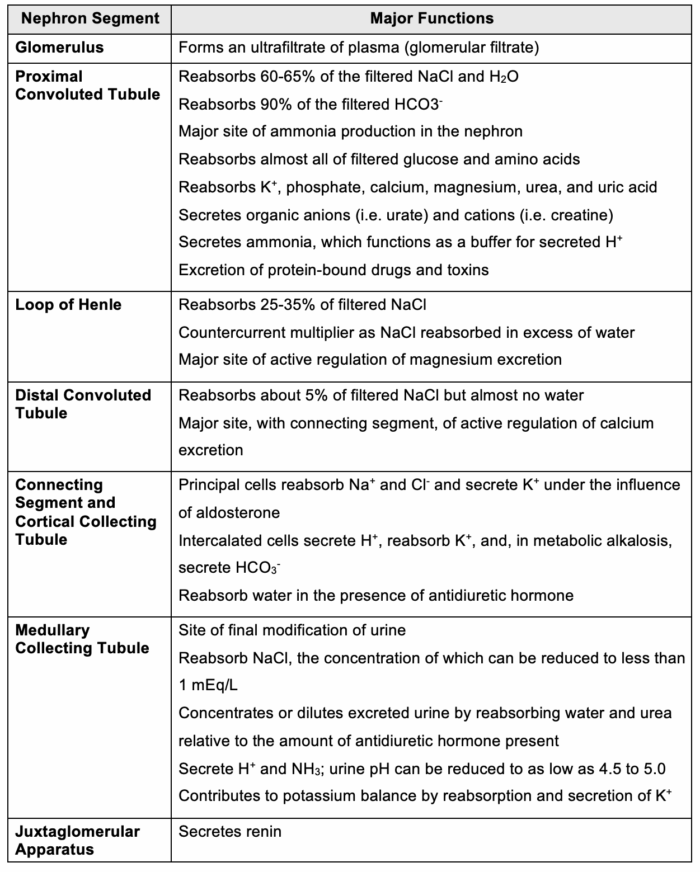
- The kidneys contain approximately one million nephrons each, which are recognized as the primary functional components. At the beginning of the nephron lies the renal corpuscle, a structure formed by the glomerulus and Bowman’s capsule, where the initial blood filtration occurs. The resulting filtrate then passes through the various tubular segments, including the proximal convoluted tubule (PCT), loop of Henle, distal tubule, collecting tubule, and the juxtaglomerular apparatus (Figure 3).3
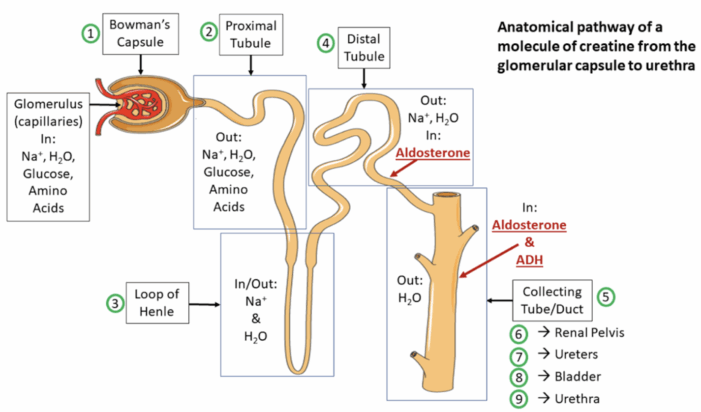
Figure 4. Anatomical pathway of a creatine molecule from the glomerular capsule to the urethra. Source: Wikipedia. Servier Medical Art. CC-BY-SA-3.0 Link
Renal Corpuscle
- Each renal corpuscle is composed of a glomerulus formed by capillary loops that project into Bowman’s capsule to provide an extensive surface for filtration.
- Blood is delivered to the glomerulus by a single afferent arteriole and exits through a single efferent arteriole. Afferent and efferent arteriolar tone play key roles in modulating filtration pressure; increased efferent tone elevates it, while increased afferent tone decreases it.3
- Filtration occurs across a three-layer barrier: fenestrated endothelial cells (70–100 nm), a fused basement membrane, and interdigitating epithelial cells forming narrow slits (~25 nm). A net negative charge across the filtration barrier facilitates the movement of cations while restricting larger and negatively charged molecules.3
- Intraglomerular mesangial cells are situated between capillaries and the basement membrane, regulating glomerular blood flow and performing phagocytic functions. Filtration is decreased when mesangial cells contract in response to factors such as angiotensin II, vasopressin, thromboxane A2, and endothelins. Relaxation of mesangial cells, promoted by agents like atrial natriuretic peptide and prostaglandin E2, leads to increased filtration.3
- Glomerular filtration pressure, averaging 60 mm Hg (approximately 60% of mean arterial pressure), is opposed by plasma oncotic pressure (~25 mm Hg) and renal interstitial pressure (~10 mm Hg).3
- Approximately 20% of plasma is filtered into Bowman’s capsule during glomerular blood flow.3
PCT
- Roughly 65–75% of filtrate in Bowman’s capsule is reabsorbed isotonically in the proximal tubule, where water and sodium are absorbed in proportion. Sodium reabsorption is the primary function of the PCT and is driven by Na⁺–K⁺-ATPase at the basolateral membrane, creating a gradient that allows passive sodium entry; this process is enhanced by angiotensin II and norepinephrine, and inhibited by dopamine and fenoldopam.3
- Sodium reabsorption in the proximal tubule is linked to the uptake of glucose, phosphate, amino acids, and the secretion of hydrogen ions, aiding in the reabsorption of about 90% of filtered bicarbonate. The Na⁺–K⁺ pump facilitates the uptake of potassium, calcium, and magnesium.3
- Chloride is reabsorbed passively or via a K⁺–Cl⁻ cotransporter, while water follows osmotic gradients through aquaporin-1 channels.
- The proximal tubule also secretes organic ions, with substances like trimethoprim inhibiting creatinine excretion.3
Loop of Henle
- The loop of Henle, comprising descending and ascending segments, creates a hypertonic interstitial environment in the medulla, which aids the collecting tubules in concentrating urine. The descending limb, a continuation of the proximal tubule, descends into the renal medulla before turning upward as the ascending limb. The ascending limb consists of thin and thick portions, with cortical nephrons having shorter loops extending into the outer medulla and juxtamedullary nephrons possessing deeper loops extending into the inner medulla.3
- Approximately 25 to 35% of the ultrafiltrate formed in Bowman’s capsule reaches the loop of Henle, where 15% to 20% of filtered sodium is reabsorbed. Reabsorption in the loop primarily occurs passively, following osmotic gradients, except in the thick ascending segments, where sodium and chloride reabsorption is active. Chloride concentration in the tubular fluid acts as a limiting factor in this process.3
- The thick ascending limb is impermeable to water, leading to the formation of hypotonic fluid as it exits, and contributing to the countercurrent multiplier mechanism. This mechanism increases both the osmolarity of the fluid and the surrounding interstitium as the fluid moves deeper into the medulla. Urea also contributes to this osmolarity.3
- The countercurrent multiplier mechanism, involving the loop of Henle, collecting tubules, and vasa recta, assists in urine concentration. Additionally, the thick ascending limb plays a key role in calcium and magnesium reabsorption, with parathyroid hormone promoting calcium uptake.3
Distal Convoluted Tubule
- The distal tubule receives hypotonic fluid from the loop of Henle and is primarily involved in minor adjustments to the tubular fluid. Due to tight junctions between tubular epithelial cells, it is relatively impermeable to water and sodium, thus helping to maintain the osmotic gradients established by the loop of Henle.3
- Only about 5% of the filtered sodium is reabsorbed in the distal tubule, with Na⁺-K⁺ ATPase activity providing the energy required for this process. Sodium reabsorption is facilitated by an Na⁺-Cl⁻ carrier on the luminal membrane and is directly related to the delivery of sodium to the segment.3
- Calcium reabsorption in this segment is predominantly regulated by parathyroid hormone and vitamin D. In the final portion of the distal tubule, known as the connecting segment, both calcium reabsorption and aldosterone-mediated sodium reabsorption occur.3
Collecting Tubule
- The collecting tubule is typically divided into cortical and medullary segments, which together contribute to the reabsorption of 5% to 7% of the filtered sodium. Differences in urea permeability between the cortical and medullary collecting tubules contribute to medullary hypertonicity. While cortical tubules allow urea passage, vasopressin increases permeability in the inner medullary tubules, leading to urea concentration and diffusion into the interstitium, enhancing tonicity.3
- Cortical Collecting Tubule
- The nephron’s collecting tubule contains two cell types: principal cells, which secrete potassium and mediate sodium reabsorption via aldosterone; and intercalated cells, responsible for acid-base regulation.3
- Principal cells maintain electrical neutrality by reabsorbing sodium and either reabsorbing chloride or secreting potassium. Aldosterone enhances Na+-K+ ATPase activity, increasing the number of open channels for sodium and potassium.3
- Intercalated cells also secrete hydrogen ions and can reabsorb bicarbonate in response to alkaline loads.3
- Medullary Collecting Tubule
- The medullary collecting tubule, influenced by antidiuretic hormone (ADH), regulates water reabsorption by expressing aquaporin-2 channels in response to dehydration, leading to concentrated urine. When hydration is adequate, vasopressin secretion is reduced, keeping the urine hypotonic.3
- This segment also contributes to urine acidification by secretion hydrogen ions as titratable acids and ammonium.3
Juxtaglomerular Apparatus
- The juxtaglomerular apparatus in each nephron includes a specialized segment of the afferent arteriole (containing juxtaglomerular cells in its wall) and the macula densa (“dense spot”), located at the end of the thick ascending loop of Henle.2,3
- The juxtaglomerular cells synthesize the enzyme renin, involved in the renin-angiotensin-aldosterone system.3 The juxtaglomerular cells are innervated by the sympathetic nervous system.
- Renin release depends on beta-1 adrenergic sympathetic stimulation, changes in afferent arteriolar wall pressure, and changes in chloride flow past the macula densa.3
- Please see the OA summary on renin-angiotensin-aldosterone system for more details. Link
The major functions of the nephron segments are summarized in Table 1.
Autoregulation
- GFR is maintained through intrinsic renal autoregulation to ensure effective filtration while preventing fluid and solute imbalance. This regulation is achieved primarily via the myogenic mechanism, which induces afferent arteriole constriction in response to increased intraluminal pressure, and tubuloglomerular feedback, which adjusts arteriole tone based on sodium delivery to the distal nephron.4
- The macula densa detects increased sodium flow, leading to ATP-mediated calcium influx, vasoconstriction, and suppression of renin release from the juxtamedullary apparatus located on the afferent nephron, ultimately reducing GFR. Conversely, reduced sodium delivery results in vasodilation and enhanced renin secretion to promote GFR restoration.4
- These mechanisms function optimally within a perfusion pressure range of approximately 80–180 mm Hg; autoregulatory efficiency is diminished outside this range.4
Perioperative Alterations in Renal Function
- Direct effects of anesthesia include changes in renal blood flow autoregulation, ADH response, and tubular transport. Regional anesthesia has a milder impact, primarily affecting hemodynamics.5
- Inhalational anesthetics, opioids, barbiturates, and benzodiazepines reduce GFR and urine output, with preoperative hydration mitigating these effects.5
- Mechanical ventilation decreases urine volume and sodium excretion, influenced by intrathoracic pressure, ADH, baroreceptor unloading, and the renin-angiotensin system.5
- Pneumoperitoneum during laparoscopic procedures can cause an abdominal compartment syndrome-like state.3 This often causes oliguria or anuria that is proportional to insufflation pressures. Proposed mechanisms include:
- Vena caval compression
- Renal parenchymal compression
- Decrease cardiac output
- Increase in renin, aldosterone, and ADH
- The only confirmed direct toxic effect associated with an anesthetic agent is fluoride-related nephrotoxicity caused by methoxyflurane.5
- Compound A, a breakdown product of sevoflurane, causes acute kidney injury in laboratory animals, especially at low fresh gas flow rates. However, no clinical study in humans has detected significant kidney injury.3
References
- Inker LA, Perrone RD. Assessment of kidney function. In: Post T, ed. UpToDate. 2024. Accessed April 17, 2025. Link
- Urinary structures and functions. In: Anatomy and Physiology. CCCOnline. Link
- Butterworth JF, Mackey DC, Wasnick JD. Chapter 30: Kidney physiology. In: Morgan and Mikhail’s Clinical Anesthesiology,7e. McGraw-Hill Medical; 2025. Accessed April 26, 2025.
- Dalal R, Bruss ZS, Sehdev JS. Physiology, renal blood flow, and filtration. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. Link
- Burchardi H, Kaczmarczyk G. The effect of anaesthesia on renal function. Eur J Anaesthesiol. 1994;11(3):163-8. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.