Copy link
Quality Improvement Methodology
Last updated: 05/24/2024
Key Points
- The Institute for Healthcare Improvement (IHI) sets quality goals, including efficient, safe, reliable, effective, and equitable care.
- Quality improvement (QI) is a science of methodical planning, multiple iterations, and interventions to yield improvements in a process or area. QI projects should follow a true scientific method and attempt to monitor improvement over time and reflect sustainability.
- Common quality improvement planning tools include Model for Improvement, Lean Six Sigma, Plan Do Study Act (PDSA), Key Driver Diagrams (KDD), and Run charts.
- Model for Improvement and Lean Six Sigma each provide different methodologies to best navigate a QI program through different tools.
- Regulatory Bodies uphold specific standards of practice and safety in the hospital and perioperative setting and may play a role in accreditation, compliance, quality improvement, and safety.
Introduction
- Over time, improvements in anesthetic care have shown a decrease in mortality related to anesthesia from 2/10,000 to less than 5/1,000,000.1
- According to the Institute of Medicine, quality goals in the perioperative arena may include but are not limited to:
- Efficient
- Safe
- Reliable
- Effective
- Equitable care2
- These quality goals may be met by:
- addressing ongoing safety issues as they arise in an organization (e.g., a sentinel event)
- meeting criteria set by regulatory bodies.
- aiming to achieve a national benchmark that is set by a regulatory body or other organization
- setting other goals to align with a hospital or organization as set forth by hospital leadership
- Institutions may have internal standards that are driven to comply with agency standards and standards that are emphasized to comply with a best practice goal for specific issues or conditions.
- QI is a science of methodical planning and multiple iterations and interventions to yield improvements in a process or area. It combines ideas of how to associate effective processes with clinical practice into practical methods to improve overall patient care. Aligning the organization’s goals and leadership priorities is also crucial for project planning.
Quality Improvement Methodology
- Change is required to improve a current process or workflow. However, not all changes in a process necessarily show improvement.
- Two popular QI methodologies include Model for Improvement and Lean Six Sigma.
- The Model for Improvement (Table 1) utilizes a foundation based on three crucial questions:
- What are we trying to accomplish?
- How will we know that a change is an improvement?
- What changes can we make that will result in improvement?3
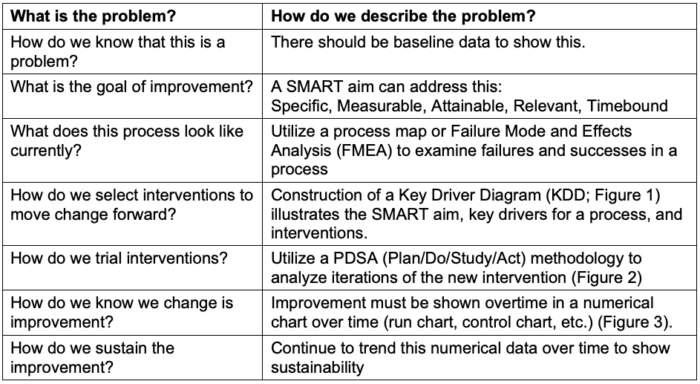
Table 1. Model for improvement questions to answer
- Tools that are used frequently in the model for improvement are:
- Key Driver Diagram: This diagram shows the entire direction of the project by stating the SMART Aim or goal of the project (left column), the key drivers (middle column), and the interventions (right column) being used to drive the desired change; key drivers are the “what” that affect the aim of the project, and interventions are the “how” to modify the key drivers of the aim (Figure 1).
- Plan/Do/Study/Act (PDSA) cycle: This represents rapid cycle interventions that are trialed to drive the desired change. An analysis (should we adapt? adopt? or abandon? the new intervention) is conducted to determine if the intervention requires another iteration before moving to the subsequent PDSA cycle (Figure 2).
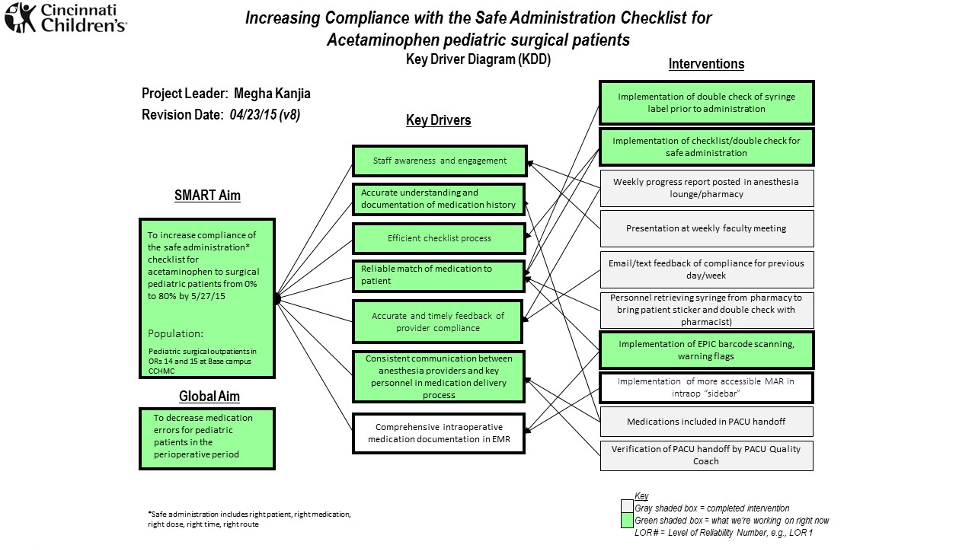
Figure 1. Example of a key driver diagram (KDD) for a QI project showing a SMART aim, key drivers, and interventions utilized for the project. A KDD shows the entire direction of the project by stating the SMART Aim or the goal of the project (left column), the Key Drivers (middle column), and the interventions (right column) being used to drive the desired change. (courtesy of Megha Kanjia)
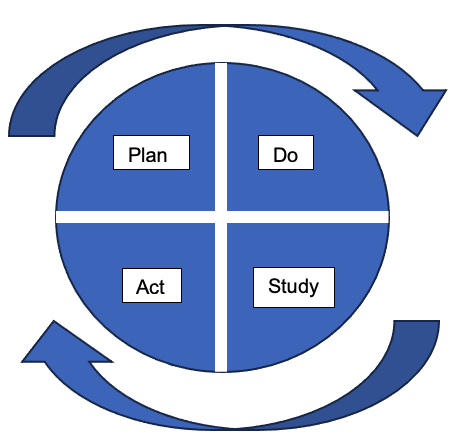
Figure 2. Plan, Do, Study, Act (PDSA) to try implementing interventions. This rapid cycle planning allows teams to analyze the current intervention by determining whether to “Adapt, Adopt, or Abandon” the new intervention prior to take the next step in the project. (courtesy of Megha Kanjia)
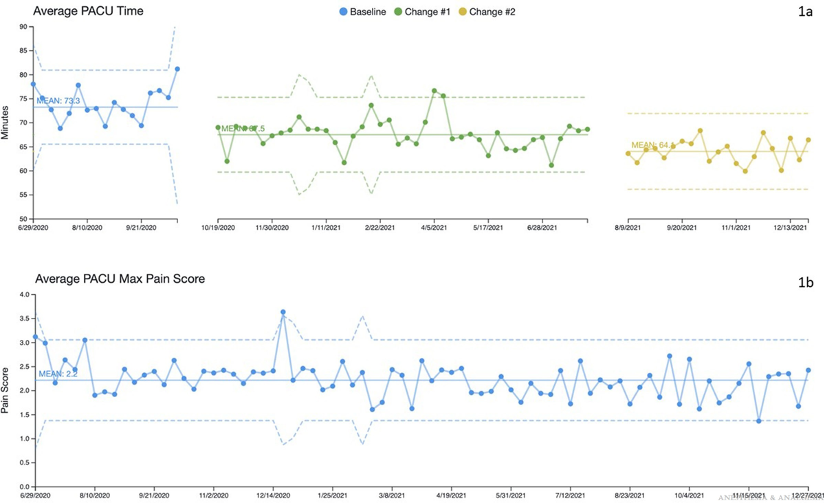
Figure 3. 1a. Example from a QI project showing change in outcome over a period of time with interventions noted at a particular date. 1b. delineates a second outcome over time that was simultaneously tracked with the first outcome being measured above. Used with permission from Martin, LD, et al. Completion of an enhanced recovery program in a pediatric ambulatory surgery center: A quality improvement initiative. Anesth Analg. 2022; 135(6):1271-1281.
- The Lean Six Sigma methodology asks, “How do we reduce waste in a system to make it more ‘lean’?”
- Lean improvement strives to eliminate waste by utilizing a “systematic approach to identifying and eliminating waste (nonvalue-added activities) through continuous improvement by flowing the product at the pull of the customer’s pursuit of perfection.”3
- Lean methodology aims to enhance effectiveness by mitigating defects and seeking errors less than 1/10^6 (to the sixth power) in frequency.
- The Lean approach is based on five major principles:
- Defining value (what a customer is willing to pay for) based on the “customer’s perspective.”
- Identifying value streams required to provide to the customer
- Making the value-added steps move smoothly
- The customers “pull” services as needed (as opposed to a “push” system)
- Everyone is pursuing “perfection.”3
Regulatory Bodies
- Regulatory bodies are governing bodies that both create and uphold specific standards of practice and safety in the hospital and perioperative setting.
- They may play a role in accreditation, compliance, and safety to help generate appropriate benchmarking metrics for hospitals to follow.
- These national benchmarks, driven by QI organizations, may lead to quality improvement projects and initiatives within a clinical setting.
- Examples of regulatory bodies in the perioperative arena:
- Joint Commission Organization
- American College of Surgeons (ACS)
- Association of Perioperative Registered Nurses
- Under the guidance of regulatory bodies (ACS), organizations such as the National Surgical Quality Improvement Program may collect a sampling of data on various patients, procedures, and other perioperative details, such as the evaluation of blood product administration in specific procedures, to help set national benchmarks.
References
- Heitmiller E, Koka R. Safety and Outcome in Pediatric Anesthesia. Davis PJ, Cladis FP. Smith’s Anesthesia for Infants and Children E-Book. Elsevier Health Sciences; 2017.
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US); 2001. PubMed
- Langley, GJ. The Improvement Guide: a Practical Approach to Enhancing Organizational Performance. Jossey-Bass, 2014. Pg 1-5, 463-465.
- Martin LD, Chiem JL, Hansen, E, et al. Completion of an enhanced recovery program in a pediatric ambulatory surgery center: A quality improvement initiative. Anesth Analg. 2022; 135(6):1271-81. PubMed
Other References
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.