Copy link
Pulmonary Embolism
Last updated: 09/09/2025
Key Points
- Pulmonary embolism (PE) is a life-threatening emergency resulting from the lodging of a thrombus in the pulmonary vasculature.
- Avoiding right ventricular failure is crucial during the downward spiral of acute PE, leading to systemic hypoperfusion and ischemia.
- Diagnosing PE in the perioperative setting is challenging and requires a high index of suspicion, as it is often a diagnosis of exclusion.
- Anesthetic management of PE includes vasopressors, pulmonary vasodilators, intravenous fluids, and adjusting airway management.
Definition and Epidemiology
- Acute pulmonary embolism (PE) is a common and potentially fatal form of venous thromboembolism (VTE) in which the thrombus travels through the venous circulation and lodges in the pulmonary arterial system.1 Most PE originate from the lower extremities, known as a deep venous thrombosis.
- The annual incidence rate of VTE ranges from 75-269 per 100,000 persons, with risk doubling with each decade of life after 40.2 While the true incidence rate of PE remains unknown, PE is responsible for 100,000 deaths every year in the United States.1
- Rates of intraoperative and postoperative PE vary and have been difficult to determine due to the common asymptomatic state and improved imaging to detect smaller, peripheral emboli. The incidence of fatal PE, however, has remained constant at 0.4 per 1000 patients per year.3
- The main risk factors for thrombosis are described by Virchow’s Triad: stasis of blood flow, vascular endothelial damage, and hypercoagulability3 (Table 1).
- General anesthesia compared to a lumbar epidural catheter is associated with an increased risk of PE. A 55% reduction of PE is seen in surgical patients with epidural catheters.3
- There is a fivefold increase in the risk of PE during and after surgery, with some of the highest rates occurring in these surgical populations.3
- Total hip arthroplasty
- Total knee arthroplasty
- Hip fracture repair
- Trauma
- Acute spinal cord injury
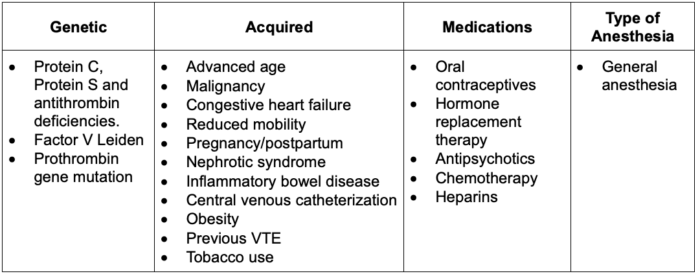
Table 1. Risk factors for PE; Abbreviation: VTE, venous thromboembolism
Pathophysiology
- Blood flow through the lower extremities relies on both laminar flow propelled by left ventricular contraction and pulsatile flow generated by skeletal muscle contraction. Disruption of pulsatile flow increases the risk of thrombus formation in venous valve pockets.
- The absence of pulsatile flow causes endothelial hypoxia in venous valve pockets, leading to an increased expression of pro-coagulation proteins and leukocyte recruitment. The platelets and leukocytes aggregate in layers into a fully formed thrombus that encroaches into the vessel until detaching.4
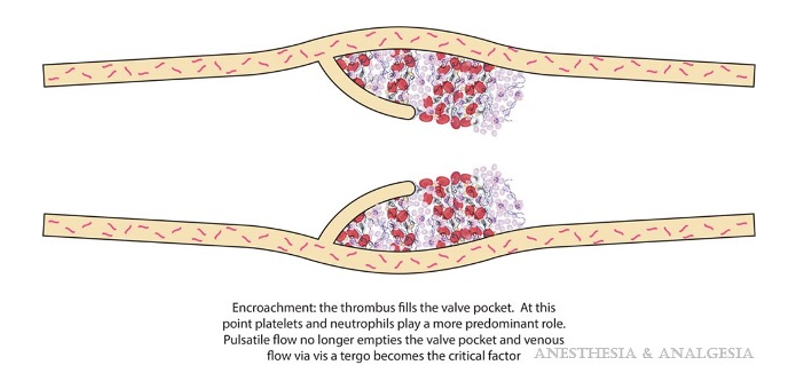
Figure 1. Fully formed thrombus ready to dislodge. Used with permission: Gordon R, et al. Perioperative venous thromboembolism: A review. Anesth Analg. 2017;125(2):403-12.
- The embolus then detaches from the valve pocket and travels through the systemic venous system, through the right-sided chambers of the heart, and lodges in the pulmonary arterial system.1
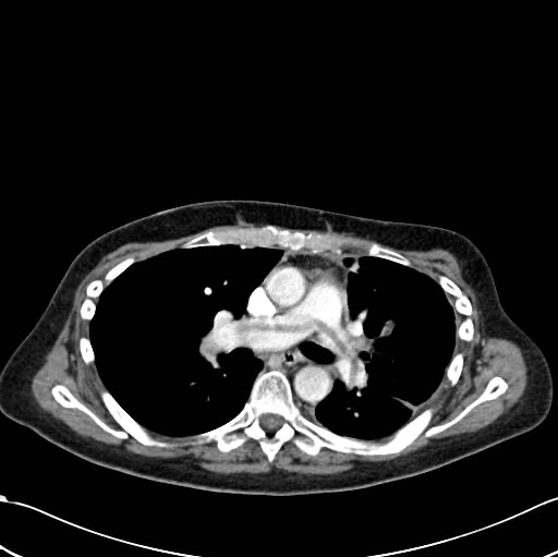
Figure 2. Saddle embolus on computed tomography. Case courtesy of Jeremy Jones, Radiopaedia.org, rID: 6120 Link
- Obstruction of pulmonary arterial supply leads to alveolar dead space and VQ mismatch, resulting in hypoxemia. As blood flow is shunted away from the obstructed vessels, the rest of the lung tissue is over-perfused, leading to edema, alveolar hemorrhage, and eventually atelectasis.1
- Hemodynamically, this leads to a sharp increase in pulmonary vascular resistance and right ventricular (RV) afterload. Due to the geometry of the right ventricle (thin myofibrils in series for increased compliance compared to the thicker left ventricle), this large pressure increase causes the body to increase right atrial pressure and RV preload to compensate and maintain cardiac output (CO).3
- Since the RV cannot endure consistently elevated pressures, it dilates and eventually bulges into the left ventricular (LV) space across the interventricular septum, decreasing filling of the LV even further.
- As the LV pumps less and less blood while the RV fails to adequately perfuse the obstructed and damaged pulmonary vasculature, CO begins to drop precipitously, and systemic hypotension follows. Additionally, the dilation of the RV, along with an ever-increasing RV afterload, leads to increased RV wall stress, and coupled with hypo-perfused coronary arteries, can precipitate ischemia and eventual myocardial infarction.3
- RV wall stress can be determined by Laplace’s Law:
Wall stress = pressure x radius / 2 x wall thickness
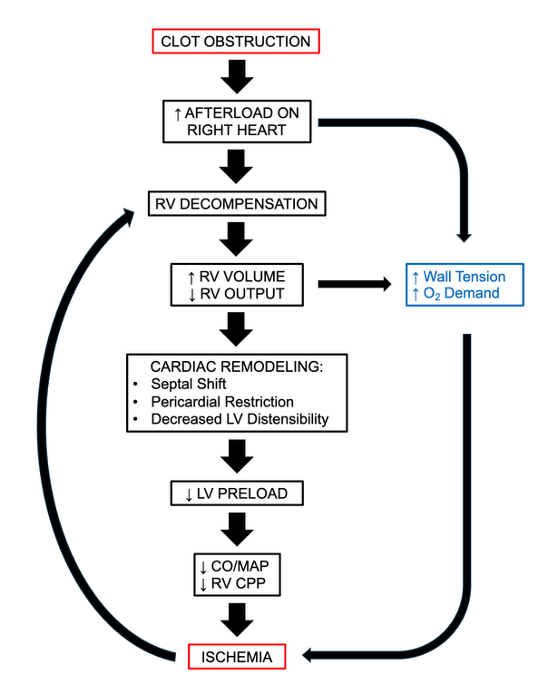
Figure 3. Pathophysiology of a major PE
Diagnosis
- The diagnosis of PE is challenging and requires a high level of clinical suspicion, especially in an intraoperative setting.
- Over half of all hospitalized patients presenting with major PE will have signs of hemodynamic instability, with 18% presenting in cardiac arrest and 10% presenting with hypotension requiring vasopressors. Other key clinical signs include dyspnea, tachycardia, elevated jugular venous pressure, and arterial hypotension with a shallow pulse.3
- PE triage stratifies patients into categories of risk based on signs of shock/hypotension, scoring on the validated Pulmonary Embolism Severity Index (PESI) Link, RV function, and cardiac biomarkers.2
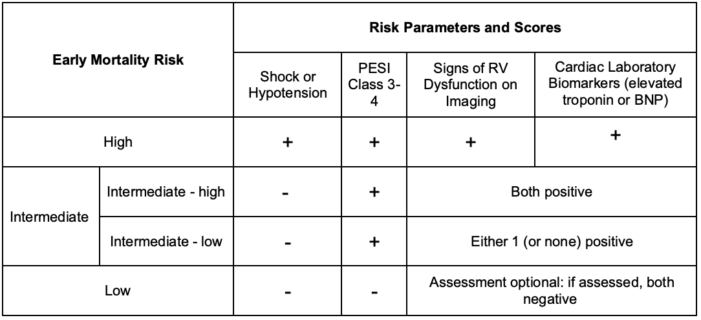
Table 2. Acute PE: Risk stratification.2 Abbreviations: BNP, brain natriuretic peptide; PESI, Pulmonary Embolism Severity Index
- Intraoperatively, PE is a diagnosis of exclusion achieved through several diagnostic methods3:
- EKG: Positive for sinus tachycardia, atrial arrhythmias, ST changes, T wave inversions, right axis deviation, and S1Q3T3 pattern for RV dysfunction.
- Arterial blood gas: hypoxemia, respiratory alkalosis, hypocapnia
- Physiologic dead space: increased
- Laboratory tests:
- D-Dimer: sensitive but not specific, use negative to rule out PE
- Troponin and brain natriuretic peptide: elevated sometimes, but not specific or sensitive
- Chest x-ray: cardiomegaly, also used to rule out other cardiopulmonary diseases
- Transesophageal echocardiography (TEE) findings in PE include:
- RV/LV end-diastolic diameter ratio >0.7
- RV/LV area ratio >0.66
- RV end-diastolic diameter >27 mm
- “McConnell sign” refers to akinesia of the midfree RV wall with preserved apical contractility5
- Septal shift
- Tricuspid regurgitation >270 cm/sec
- Outside of surgery, V/Q scans and single-photon emission computed tomography (SPECT) are first-line tests. Angiography is the gold standard test, but it is expensive and invasive.3 Spiral CT has a high sensitivity for detecting PE in central arteries.
Anesthetic Considerations and Perioperative Management
- Upon recognizing or being highly suspicious of a PE, one should immediately notify the surgical team and call for help to implement the following cascade of actions:
- Administer 100% oxygen.
- Vasopressors and/or inotropes may be necessary for hemodynamic instability.
- Consider a modest fluid bolus.
- Insert an arterial catheter for blood draws.
- Adjust the ventilator settings depending on the arterial blood gas.
- Consider a TEE.
- Consider extra-corporeal membrane oxygenation in dire cases for specific patient populations.
- Vasopressor/Inotrope Choice:
- The agent used should improve RV function, maintain blood pressure (BP), and coronary perfusion pressure (CPP) in the setting of a fixed obstruction to blood flow.
- Norepinephrine is the agent of choice due to α-1 mediated vasoconstriction, which increases BP, RV CPP, and venous return. β1 stimulation by norepinephrine improves RV and LV contractility and CO.3
- Vasopressin is also a helpful agent, which will increase BP and RV ejection fraction.6
- Inotropes in general may not be beneficial. They increase CO through an increased heart rate, stroke volume, or both, which increases oxygen consumption. Inotropes cause tachycardia and sometimes arrhythmias, which would decrease the time for passive RV Filling and potentially loss of atrial kick.6
- While phenylephrine does increase BP, norepinephrine’s activity is superior, and phenylephrine also worsens RV function.6
- Pulmonary Vasodilators:
- Inhaled nitric oxide decreases pulmonary artery pressure, which will increase cardiac output and improve RV function and pulmonary gas exchange.3
- Supplemental oxygen is also a pulmonary vasodilator.
- Fluid Management
- Fluids should be used judiciously as volume overload can lead to over-distension of the RV and decrease cardiac output.
- A fluid challenge of 500 mL over 15-30 min can be given to patients with low central venous pressure.7
- Ventilator Settings:
- Positive pressure ventilation can cause a decrease in venous return. Maintain settings at 6-8ml/kg ideal body weight and be cautious of using positive end-expiratory pressure.6
- TEE:
- While capturing a visible embolus on TEE is rare, it can help assess fluid status as well as establish an alternative diagnosis.
- RV dilation is the most common finding, indicating at least a 30% obstruction of the pulmonary vasculature.3
Outcomes
- Even with anticoagulation and thrombolytic therapy, mortality can be up to 32%.
- Risk factors for increased mortality include age greater than 70 years, congestive heart failure, chronic obstructive pulmonary disease, and higher American Society of Anesthesiologists physical status.3
References
- Turetz M, Sideris A, et al. Epidemiology, pathophysiology, and natural history of pulmonary embolism. Semin Intervent Radiol. 2018;35(2):92-98. PubMed
- Konstantinides S, Barco S, et al. Management of pulmonary embolism. J Am Coll Cardiol. 2016;67(8):976-90. PubMed
- Desciak M, Martin D. Perioperative pulmonary embolism: diagnosis and anesthetic management. J Clin Anesth. 2011;23(2):153-65. PubMed
- Gordon R, Lombard F. Perioperative venous thromboembolism: A review. Anesth Analg 2017;125(2):403-12. PubMed
- McConnell MV, Solomon SD, et al. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78(4):469-73. PubMed
- McGuire WC, Sullivan L, Odish MF, et al. Management strategies for acute pulmonary embolism in the ICU. Chest. 2024;166(6):1532-45. PubMed
- Cruz G, Pedroza S, Giraldo M, et al. Intraoperative circulatory arrest secondary to high-risk pulmonary embolism. Case series and updated literature review. BMC Anesthesiology. 2023;23(1):415. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.