Copy link
Prader-Willi Syndrome
Last updated: 04/29/2025
Key Points
- Prader-Willi syndrome (PWS) is a rare genetic disorder characterized by infantile hypotonia, followed by hyperphagia and obesity, endocrinopathies, and behavioral challenges in childhood to adulthood.
- Obesity and its sequelae are a major cause of morbidity and mortality. However, growth hormone replacement therapy has shown significant benefit in weight control and surgical outcomes.1
- Hypotonia, increased aspiration risk, central and obstructive apnea, restrictive lung disease, and possible abnormal response to GABAergic anesthetics predispose patients to respiratory complications in the perioperative setting.
- Other anesthetic concerns include unreliable nil per os (NPO) status, possible difficult airway or vascular access, temperature instability, scoliosis, decreased pain sensitivity, and tantrums or compulsive behaviors.
Introduction
- PWS is a rare genetic disorder characterized by infantile hypotonia, followed by hyperphagia and obesity, endocrinopathies, and behavioral challenges in childhood to adulthood.
- PWS is caused by the absence of paternal chromosome 15q11-q13 region expression, where maternal imprinting (epigenetic silencing) occurs.1
- The most common mechanism is paternal 15q11-q13 region deletion, occurring in 60% of cases. However, PWS can also occur via uniparental disomy 15 (both chromosome 15 are maternally inherited) or defects of the paternal imprinting center.1
- The prevalence is 1 in 10,000-20,000 individuals, equally affecting males and females.1
- Currently, there is no standard screening test for PWS in newborns. In 1993, consensus diagnostic criteria were developed for the clinical diagnosis of PWS based on major and minor criteria.2
- The criteria were revised in 2001 to reflect the most sensitive criteria to prompt definitive genetic testing.3
- DNA methylation analysis confirms the diagnosis. However, further testing is required to distinguish the specific genotype abnormality.1
- Management with a multidisciplinary team, including pediatrics, clinical genetics, endocrinology, behavioral/learning experts, and dietitians, is vital. Early initiation of growth hormone replacement therapy is a cornerstone of pharmacotherapy.2
- PWS is the most common genetic cause of life-threatening obesity.1
- The most common reported cause of death is respiratory failure (31%), followed by cardiac causes (16%).1 Death typically occurs around age 30, but weight control can lead to a near-normal life span.1
Clinical Presentation and Associated Comorbidities
Evolving Clinical Presentation with Age
- The clinical presentation of PWS was traditionally divided into two nutritional stages, infantile hypotonia and poor feeding followed by childhood-adulthood hyperphagia leading to obesity. Newer research suggests a more nuanced progression with up to seven nutritional phases.2
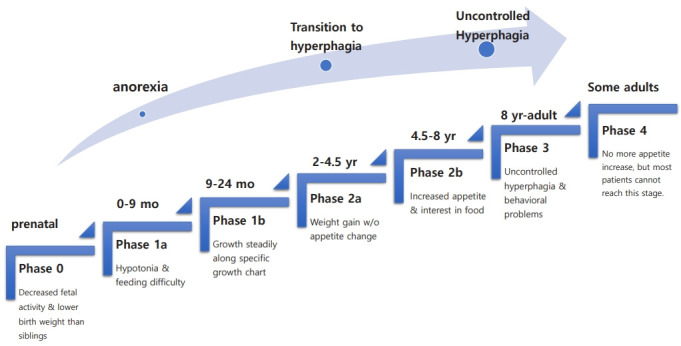
Figure 1. Nutritional phases in Prader-Willi syndrome. Source: Kim SJ, et al. Ann Pediatr Endocrinol Metab. 2001. 4 CC BY-NC 4.0
Manifestations and Comorbidities by Organ System
Respiratory
- Obstructive sleep apnea is common due to obesity, upper airway hypotonia, and adenotonsillar hypertrophy.1
- Central apnea and sleep disordered breathing due to hypothalamic dysfunction can cause desaturations. Blunted respiratory response to hypoxia and hypercarbia can exacerbate hypoventilation.5,6
- Restrictive ventilatory impairment can occur due to respiratory muscle weakness, obesity, and scoliosis.5
- Decreased salivary secretion can lead to dental caries and poor oral hygiene.1
- Decreased cough, hyperphagia, and gastrointestinal dysmotility predispose to choking and aspiration. Up to 50% of patients have recurrent respiratory infections.2
Musculoskeletal
- PWS presents in utero with reduced fetal activity, growth restriction, and a higher incidence of polyhydramnios.2
- Infantile hypotonia can lead to weak cry, diminished reflexes, poor sucking and failure to thrive.2
- Hypotonia improves with age but does not normalize. Adults have decreased tone and muscle mass. Muscle biopsy is generally normal, indicating a central cause.2
- Craniofacial features include dolichocephaly, narrow bifrontal diameter, almond-shaped eyes, and a thin upper lip with a downturned mouth.1
- Strabismus is present in 60-70% of patients.2
- Scoliosis is seen in about two-thirds of patients with a bimodal presentation at birth and early adolescence.1
Endocrine
- Hyperphagia is believed to result from an abnormal hypothalamus, causing a lack of satiety. Weight gain begins around age two. Food-seeking behaviors such as hoarding, stealing, and consuming nonedible objects are commonly observed.1
- Growth hormone (GH) deficiency is a hallmark of PWS and can lead to osteoporosis, decreased muscle mass, and short stature. Early GH replacement therapy has been shown to normalize height, decrease fat mass, and is associated with improvement in developmental milestones.2
- Hypothyroidism and central adrenal insufficiency are less common, presenting in about 10% of patients.1
- Hypogonadism is evident at birth in all individuals, presenting as genital hypoplasia and incomplete pubertal development.2
Neurologic
- Decreased pain sensitivity has been documented in PWS.1
- Several GABA receptor subunit genes reside in the deleted 15q11-q13 region, potentially leading to abnormal neurotransmission.1
- Temperature instability, particularly hypothermia, is common in childhood. Patients with PWS may not have a febrile response to infection.1
- Increased risk of seizures is present, affecting 10-20% of PWS patients.2
Psychiatric/Behavioral
- Developmental delay affects both motor and language; intellectual disability is typically mild to moderate.1
- As children enter school age, characteristic temper tantrums, aggression, compulsive behavior, including self-injury and skin picking, and cognitive rigidity develop.1,2
- PWS increases the risk of psychiatric mood disorders, including anxiety, depression, psychosis, and bipolar disorder.1
Cardiovascular
- Obesity can lead to hypertension and (particularly right-sided) heart failure.1
- Autonomic nervous system dysfunction can predispose to abnormal baroreflexes.1
Perioperative Anesthetic Considerations
No specific set of recommendations have been put forth, however several anesthetic considerations come from multiple case reports.
Preoperative Considerations
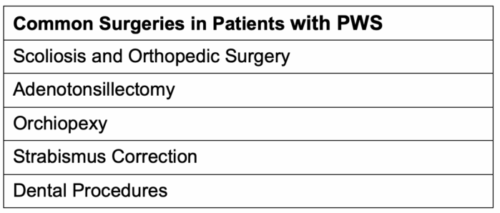
Table 1. Common surgical interventions in patients with PWS.1,7
- Preoperative physical exam should include assessment of craniofacial abnormalities (micrognathia, small mouth opening) and signs of chronic aspiration or respiratory infection.
- The patient’s medication list for hormone replacement therapies, including growth hormone, sex steroids, thyroid hormone, and glucocorticoids, should be reviewed.
- Obesity and behavioral challenges may increase the difficulty of vascular access.
- Preoperative sedation or anxiolysis that does not cause respiratory compromise should be used with caution in the setting of central and obstructive apneas.
- NPO status should be carefully assessed and confirmed with caregivers due to hyperphagia. Assume a full stomach if careful monitoring of food intake is not confirmed.
Intraoperative Considerations
- Plan for a possible difficult airway. Risk factors include micrognathia, small oropharynx or nasopharynx, obesity, and thick sticky saliva.
- Consider a modified rapid sequence induction and have a high level of suspicion for aspiration events.
- Prolonged effect of nondepolarizing neuromuscular blockers can occur, especially in infants with more severe hypotonia.8 Avoidance of neuromuscular blockade can reduce the risk of respiratory complications. However, case reports describe the use of succinylcholine and adequate reversal of rocuronium with sugammadex without complications.8,9
- Decreased GABA receptor subunits can alter the response to intravenous (IV) anesthetics.1 Electroencephalography may allow for more accurate dose titration of GABAergic drugs.8
- Scoliosis typically presents with lumbar or thoracolumbar curves, which can present a challenge when placing neuraxial anesthesia.1
- Temperature instability may be exacerbated under anesthesia. Other causes of temperature instability, including infection or hypothyroidism, should be ruled out.
- Consider administration of stress dose steroids during critical illness or prior to surgery unless central adrenal insufficiency has been ruled out.1
- Avoidance of atropine is recommended to avoid worsening thick, adherent saliva.7
Postoperative Considerations
- Obstructive sleep apnea, hypotonia, and central apnea may contribute to postoperative hypercarbia and desaturation events. Consideration should be given for overnight observation on a case-by-case basis.8
- Hypersomnolence due to hypothalamic dysfunction and slow emergence from anesthesia can prolong postoperative recovery time.1
- A high pain threshold and difficulty communicating may lead to undertreated pain and mask post-operative complications.
- Skin picking at incision sites can lead to surgical complications.1

Table 2. Perioperative considerations for general anesthesia in patients with PWS. Abbreviations: NPO = nil per os, NMB= neuromuscular blocker, EEG = electroencephalography.
References
- Butler MG, Miller JL, Forster JL. Prader-Willi syndrome: clinical genetics, diagnosis and treatment approaches: an update. Curr Pediatr Rev. 2019;15(4):207-218. PubMed
- Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14(1):10-26. PubMed
- Gunay-Aygun M, Schwartz S, Heeger S, et al. The changing purpose of Prader-Willi syndrome clinical diagnostic criteria and proposed revised criteria. Pediatrics. 2001;108(5):e92. PubMed
- Kim SJ, Cho SY, Jin D-K. Prader-Willi syndrome: an update on obesity and endocrine problems. Ann Pediatr Endocrinol Metab. 2021;26(4):227-36. PubMed
- Tan HL, Urquhart DS. Respiratory complications in children with Prader-Willi syndrome. Paediatr Respir Rev. 2017; 22:52-9. PubMed
- Cataletto M, Angulo M, Hertz G, Whitman B. Prader-Willi syndrome: A primer for clinicians. Int J Pediatr Endocrinol. 2011;2011(1):12. PubMed
- Dearlove OR, Dobson A, Super M. Anaesthesia and Prader-Willi syndrome. Paediatr Anaesth. 1998;8(3):267-271. PubMed
- Kim KW, Kim SH, Ahn EJ et al. Anesthetic management with a neuromuscular relaxant and sugammadex in a patient with Prader–Willi syndrome: A case report. SAGE Open Med Case Rep. 2020; 8:2050313X20927616. PubMed
- Jain A, Bala I, Makkar JK. Anesthetic management of Prader–Willi syndrome: what if neuromuscular relaxants could not be avoided? J Anesth. 2012;26(2):304-5. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.