Copy link
Postpartum Hemorrhage: Management
Last updated: 06/03/2025
Key Points
- Detection of postpartum hemorrhage (PPH) requires continuous surveillance for early warning signs, quantitative blood loss (QBL) measurements, and effective team communication.
- Obstetric massive transfusion protocols (MTPs) should include a fibrinogen source to treat coagulopathy along with uterotonic and antifibrinolytic medications.
- Point-of-care viscoelastic testing can detect coagulopathy and inform transfusion decisions.
Introduction
- PPH is defined as blood loss greater than one liter or active bleeding with hypovolemia in the first 24 hours after delivery, regardless of delivery mode.1
- 11% of maternal deaths in the United States are associated with PPH.2
- 54-93% of deaths due to PPH are likely preventable.1,2
- 70-80% of PPH is due to uterine atony.2
- Transfusion for PPH is required in approximately 40 per 10,000 deliveries.2
- Up to 46% of PPH occurs in low-risk patients with no identifiable risk factors.3
- PPH remains a leading cause of maternal morbidity and mortality due to a delay in recognition and management.2
- Management of PPH requires the timely detection, source control, and identification of coagulopathy. Swift crisis response is crucial for low-risk patients who experience unexpected bleeding.3
- QBL is superior to visual estimation in preventing late recognition of PPH due to underestimation of cumulative blood loss.4
Use of Staged Protocols to Manage PPH
- Efforts by the California Maternal Quality Care Collaborative have lowered PPH morbidity.
- The 2014-18 California Pregnancy-Associated Mortality Review of PPH deaths determined that 63.3% remained highly preventable.3
- Management of PPH can be organized into categories of readiness, recognition, response, and reporting.
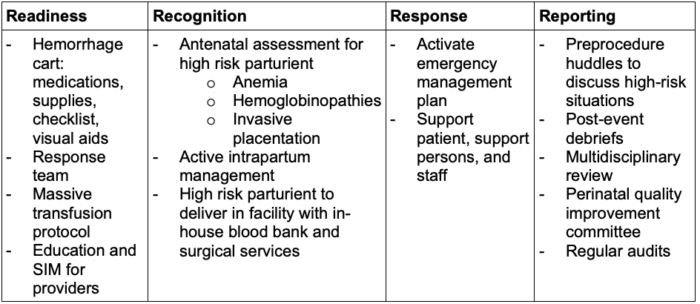
Table 1. Categories of PPH management with examples
- Staged PPH protocols are used to activate a MTP and guide medication administration and laboratory testing.5
- Objective data (maternal vital signs, QBL) should be used to minimize bias and prevent delays in management.
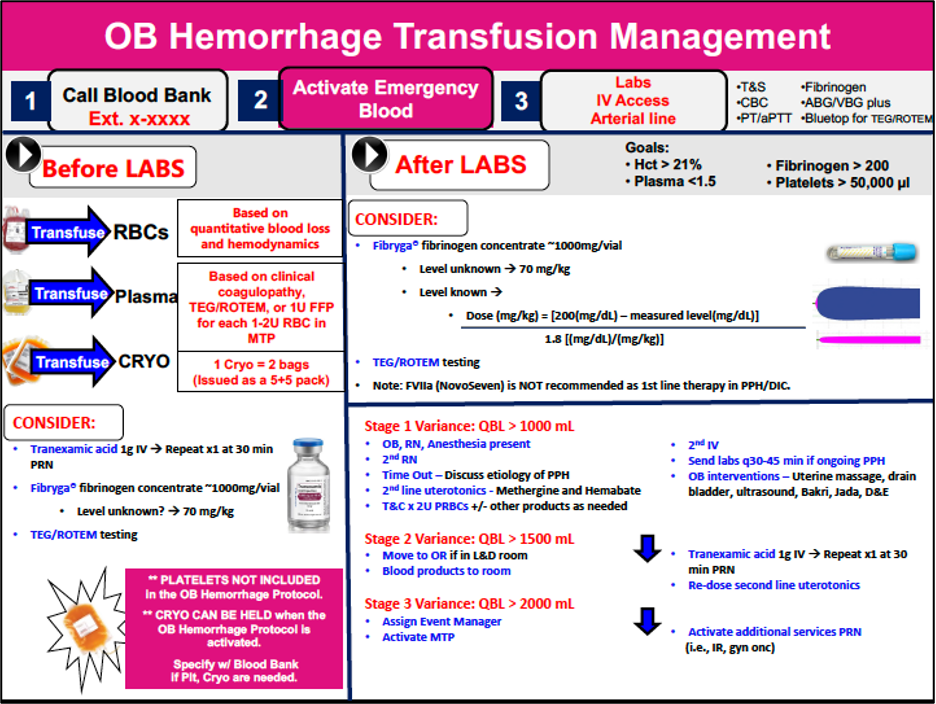
Figure 1. Example of a staged obstetric hemorrhage transfusion protocol (Brigham and Women’s Hospital, reproduced with permission)
Clinical Assessment During PPH
- Prompt detection of PPH minimizes associated morbidity and mortality.
- Clinical signs of PPH include hypotension and tachycardia but may not occur until there is more than 1.5 liters of blood loss.5
- Obstetric early warning systems help alert clinicians about clinical decompensation and decrease the time to intervention.
- Both risk assessment and early warning tools should be used in combination to lower PPH morbidity.6
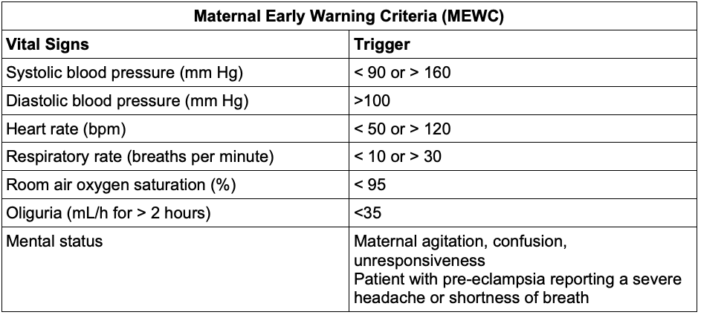
Table 2. Example of a Maternal Early Warning Criteria (MEWC). Adapted from Myhre et al. Obstet Gynecol. 2014.7
- QBL measurements may help to increase the accurate and early detection of PPH.4
- Methods for QBL include weighing sponges (gravimetry), using calibrated drapes and suction canisters, and colorimetric scanning algorithms.4
- Initiating a QBL system increases recognition of PPH and may facilitate earlier intervention; however, more studies are needed.8
- In addition to early identification of PPH, team communication to identify PPH etiology enables tailored and targeted management.
- The 4 most common causes of PPH are uterine atony, reproductive tract trauma, retained products of conception, and coagulopathy.2
- Uterine atony accounts for up to 80% of PPH and should be actively managed using uterotonics, bimanual uterine massage, and uterine compression.
- Escalation for refractory PPH can include uterine artery embolization, aortic compression or occlusion, and hysterectomy.2
- Methylergonovine and carboprost have equal efficacy as secondary uterotonics.9
- Prophylactic administration of calcium chloride may benefit patients with atonic hemorrhage.1
- Please see the OA summary on Tocolytics and Uterotonics for more details. Link
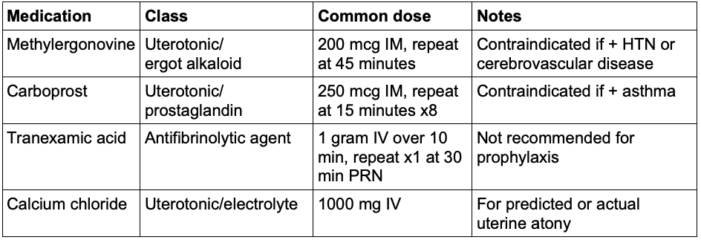
Table 3. Common medications used for uterine atony. mcg, micrograms; IM, intramuscular; HTN, hypertension; IV, intravenous.
Treatment of PPH
- PPH may occur suddenly and require massive transfusion, defined as greater than 10 units of packed red blood cells over 24h.5
- Coagulopathy is rare during PPH (15-20% cases), but hypofibrinogenemia or disseminated intravascular coagulation compounds morbidity.11
- An ideal obstetric MTP includes a fibrinogen concentrate or cryoprecipitate to manage coagulopathy rapidly.
- Point-of-care coagulation testing (thromboelastography, TEG; rotational thromboelastometry, ROTEM; sonorrheometry, Quantra) enables tailored transfusion through the assessment of global hemostasis, fibrinogen’s contribution to clot strength, and hyperfibrinolysis within 10-15 minutes.11
- Clinical factors such as mean arterial pressure, mental status, and urine output are used to confirm the adequacy of peripheral perfusion.
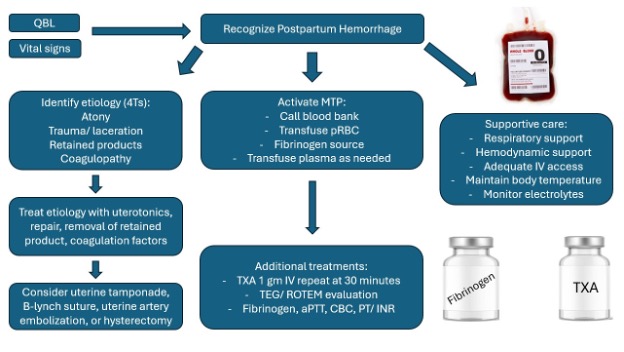
Figure 2. Proposed treatment algorithm for postpartum hemorrhage
In conclusion, until PPH ceases to be the leading cause of preventable maternal morbidity and mortality, further focus on all aspects of management is warranted.
- Earlier diagnosis and treatment of clinical deterioration are needed.
- Early detection can be optimized by focusing on QBL and activating specialized obstetric MTPs.
- Detection and replacement of low fibrinogen is a mainstay of transfusion therapy.
- Effective team communication to define and target specific PPH etiologies can facilitate management and expedite resolution.
References
- Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 183: Postpartum hemorrhage. Obstet Gynecol. 2017;130(4):e168-e186. PubMed
- Bienstock JL, Eke AC, Hueppchen NA. Postpartum hemorrhage. N Engl J Med. 2021;384(17):1635-45. PubMed
- Ladfors LV, Butwick A, Stephansson O. A validation of the California Maternal Quality Care Collaborative obstetric hemorrhage risk assessment tool in a Swedish population. Am J Obstet Gynecol MFM. 2024;6(1):101240. PubMed
- Quantitative blood loss in obstetric hemorrhage: ACOG Committee Opinion, Number 794. Obstet Gynecol. 2019;134(6):e150-e156. PubMed
- Kogutt BK, Vaught AJ. Postpartum hemorrhage: Blood product management and massive transfusion. Semin Perinatol. 2019;43(1):44-50. PubMed
- Ende HB. Risk assessment tools to predict postpartum hemorrhage. Best Pract Res Clin Anaesthesiol. 2022;36(3-4):341-8. PubMed
- Mhyre JM, D'Oria R, Hameed AB, et al. The maternal early warning criteria: a proposal from the National Partnership for Maternal Safety. Obstet Gynecol. 2014;124(4):782-6. PubMed
- Katz D, Wang R, O'Neil L, et al. The association between the introduction of quantitative assessment of postpartum blood loss and institutional changes in clinical practice: an observational study. Int J Obstet Anesth. 2020; 42:4-10. PubMed
- Cole NM, Kim JJ, Lumbreras-Marquez MI, et al. Second-line uterotonics for uterine atony: A randomized controlled trial. Obstet Gynecol. 2024;144(6):832-41. PubMed
- Ansari JR, Yarmosh A, Michel G, et al. Intravenous calcium to decrease blood loss during intrapartum cesarean delivery: A randomized controlled trial. Obstet Gynecol. 2024;143(1):104-12. PubMed
- Katz D, Farber M, Getrajdman C, et al. The role of viscoelastic hemostatic assays for postpartum hemorrhage management and bedside intrapartum care. Am J Obstet Gynecol. 2024;230(3S):S1089-S1106. PubMed
Other References
- Postpartum Hemorrhage. American College of Obstetricians and Gynecologists PPH Practice Bulletin. Created October 2017. Accessed June 3, 2025. Link
- What We Do page. California Maternal Quality Care Collaborative. Accessed June 3, 2025. Link
- Ende H. Risk stratification of postpartum hemorrhage. OA-SOAP Fellows Webinar Series. Created November 1, 2020. Accessed June 3, 2025. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.