Copy link
Postobstructive Pulmonary Edema
Last updated: 07/08/2025
Key Points
- Postobstructive pulmonary edema (POPE) or negative-pressure pulmonary edema (NPPE) after general anesthesia commonly presents as the immediate onset of respiratory distress after relief of an airway obstruction.
- POPE should be included in the differential diagnosis of acute postoperative respiratory distress, especially those associated with radiologic changes of pulmonary edema.
- Initial treatment modalities include supplemental oxygen with the addition of positive end-expiratory pressure (PEEP) or continuous positive airway pressure (CPAP).
- With proper treatment, most cases of POPE resolve within 12-24 hours.
Introduction
- POPE or NPPE is a well-described cause of acute respiratory failure after an inspiratory effort against an obstructed airway.1
- POPE is defined as the sudden onset of fluid buildup in pulmonary alveoli that occurs as a complication of upper airway obstruction.1
- An attempt to inhale against an upper airway obstruction is theorized to result in highly negative intrathoracic pressure, causing increased venous return, decreased cardiac output, and fluid transudation into the alveolar space.2
- High negative inspiratory pressure and/or increased permeability of the alveolar capillary membrane are thought to be the main contributing factors facilitating fluid buildup; however, the exact mechanism is not well defined.2
Epidemiology
- The incidence of POPE has been reported to be as high as 1 in 1000 general anesthetic cases (0.1%) and commonly presents as acute respiratory distress that requires immediate intervention.3
- The most common risk factors are head and neck surgery, young age, and male sex.3
Pathophysiology
- Due to upper airway obstruction, most commonly laryngospasm, a significant, negative intrathoracic pressure is generated by the patient’s effort to breathe.4
- The negative pressure causes an increase in venous return and, thus, an increase in right ventricular preload. This pressure also causes a decrease in extramural hydrostatic pressure in the alveoli4 (Figure 1).
- The result is right ventricle dilation, intraventricular septum shift to the left, and left ventricular diastolic dysfunction. The dilated right ventricle and intraventricular septum shift impede diastolic filling of the left ventricle.4
- These conditions enhance pulmonary microvascular intramural hydrostatic pressure.4
- As a result, a gradient develops for fluid to flow from areas of greater pressure to lower pressure, out of the capillaries and into the lung interstitium. This results in pulmonary edema4 (Figure 1).
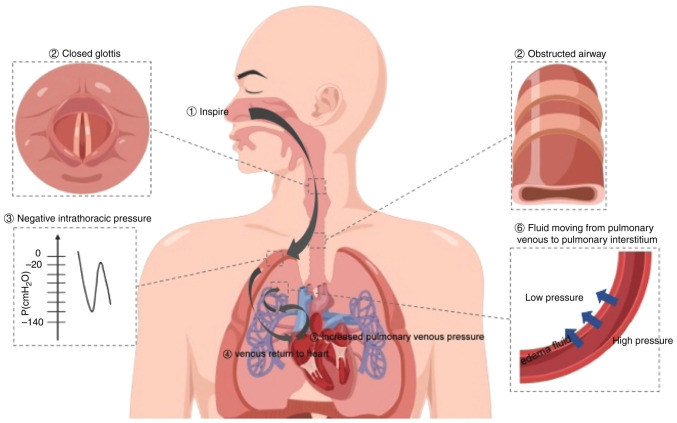
Figure 1. Pathophysiology of POPE. Source: Exp Ther Med. 2023.6 CC BY NC ND.
Clinical Presentation
- POPE after general anesthesia commonly presents as the immediate onset of respiratory distress after relief of an airway obstruction.4 Signs of upper airway obstruction include stridor, respiratory distress, paradoxical chest movement, and use of accessory breathing muscles.6
- A typical scenario leading to POPE is acute airway obstruction due to laryngospasm.7
- While laryngospasm is the most reported cause of POPE, other causes include endotracheal tube obstruction, bilateral vocal cord paralysis, goiter, acromegaly, and epiglottitis.4
- The time window from the onset of airway obstruction to the development of pulmonary edema symptoms was a few minutes in most reported cases.7
- POPE typically presents with symptoms such as dyspnea, agitation, cough with pink frothy sputum, and chest pain. Patients may describe chest pain as chest tightness or discomfort.8
- Signs of POPE include tachypnea, tachycardia, rales, and rhonchi.4
- An additional sign of POPE is decreased oxygen saturation.8 Occasionally, there may only be a slight decrease in pulse oximetry, making POPE more difficult to diagnose.6
- A chest radiograph may reveal diffuse, infiltrative shadowing in both lungs in a butterfly distribution.8
- Computed tomography scans provide more detailed images, and bilateral, diffuse ground-glass opacities and alveolar infiltrates may be present.8
- Bronchoscopy may demonstrate bloody sputum and congestion plaque in the mucosa.7
- Most cases of POPE resolve within 12-24 hours, likely due to the absence of persistent hydrostatic stress.1
Differential Diagnosis
- POPE should be included in the differential diagnosis of acute postoperative respiratory distress, especially those associated with radiologic changes, along with the following diagnoses.4
- Aspiration pneumonitis
- Radiologic changes often lag behind clinical signs, while POPE has a rapid onset of radiologic changes.4
- Direct laryngoscopy or fiberoptic bronchoscopy can help confirm the diagnosis of particulate aspiration.6
- Iatrogenic volume overload
- A review of the intraoperative record of fluid administration can provide more information.7
- If a central venous catheter is present, central venous pressure and pulmonary capillary wedge pressure can account for circulating hypervolemia.6
- Cardiogenic pulmonary edema
- This diagnosis is more likely in patients with a history of cardiac disease or significant cardiac risk factors such as hypertension, diabetes, and obesity.6
- Physical examination findings of a gallop or murmur may be present.4
- POPE often shows prominent bilateral perihilar alveolar infiltrates, while cardiogenic pulmonary edema infiltrates follow a more interstitial pattern.6
- Postoperative cardiac enzyme levels and echocardiogram may help to further distinguish cardiogenic pulmonary edema from POPE.7
- Anaphylaxis, neurogenic pulmonary edema, re-expansion pulmonary edema, and acute respiratory distress syndrome6 are other diagnoses that should be considered.4,7 The overall clinical picture is important as the diagnosis of POPE during the perioperative period can be challenging.8
- A patient’s recovery trajectory can aid in the diagnosis of POPE. It is usually self-limiting, with symptoms improving within 48 hours with appropriate treatment, and most patients fully recover within 72 hours.8
Treatment
- Relieving airway obstruction is the primary step in treating POPE.7
- Treatment goals focus on maintaining a patent airway and ensuring adequate oxygenation.4
- Initial treatment modalities include supplemental oxygen with the addition of PEEP or CPAP. Physical examination, pulse oximetry, and arterial blood gas samples help guide management.4
- PEEP and CPAP often lead to rapid resolution of symptoms.4
- An initial PEEP of 5-10 cm H20 is recommended to improve oxygenation, lung compliance, and ventilation-perfusion mismatch.4
- In severe cases, mechanical ventilation with PEEP and 100% oxygen may be required.8
- The role of diuretics in POPE is uncertain as they may exacerbate the hypovolemia and hypoperfusion in surgical patients.4
- Diuretics are still heavily debated due to their controversial role in POPE, and there is no explicit decision regarding their use.7
- Adrenocorticoid therapy has been used in the past but is not currently supported or recommended as it does not address the underlying mechanism of POPE.6
- Some studies have shown that beta-agonists might increase alveolar fluid clearance to alleviate the symptoms of pulmonary edema even if the obstruction is not caused by bronchospasm.7
- Rescue therapies for severe and refractory hypoxemia should be considered early and may include tracheotomy to bypass upper airway obstruction, neuromuscular blockade, prone positioning, and extracorporeal membrane oxygenation.1
Prevention
- Recognize risk factors for POPE, such as obesity with obstructive sleep apnea, oropharyngeal, head, and neck surgery, and younger, particularly healthy males.6-8
- Vigilantly monitor high-risk patients, particularly those undergoing high-risk surgeries.8
- Consider potential triggers such as tracheal mucus secretions, difficult airway intubation, upper airway and mediastinal tumors, compression of the thyroid isthmus, and glossoptosis during recovery from anesthesia.8
- Maintain an adequate depth of anesthesia during airway manipulation.8
- If multiple intubation attempts are made, a dose of dexamethasone may be administered before extubation to reduce laryngeal edema.6
- Thoroughly suction oropharyngeal secretions before extubation to reduce the risk of laryngospasm.6
- Ensure complete reversal of neuromuscular blockade before extubation.8
- Avoid premature extubation to decrease the risk of laryngospasm and glossoptosis.8
- Ensure a patient with risk factors is awake prior to extubation.6
- Utilize airway adjuncts, such as oropharyngeal or nasopharyngeal airways, to maintain airway patency.8
- In cases of partial obstruction, applying CPAP or noninvasive ventilation can prevent the generation of excessive negative intrathoracic pressure.8
- Recognize early signs of airway obstruction, such as stridor and paradoxical breathing, for prompt intervention.8
- Timely recognition, educating perioperative teams about POPE, and management protocols may further enhance prevention efforts.8
References
- Bhattacharya M, Kallet RH, Ware LB, et al. Negative-pressure pulmonary edema. Chest. 2016;150(4):927-933. PubMed
- Herrick IA, Mahendran B, Penny FJ. Postobstructive pulmonary edema following anesthesia. J Clin Anesth. 1990;2(2),116–120. PubMed
- Fremont RD, Kallet RH, Matthay MA, Ware LB. Postobstructive pulmonary edema: a case for hydrostatic mechanisms. Chest. 2007;131(6):1742-1746. PubMed
- Udeshi A, Cantie SM, Pierre E. Postobstructive pulmonary edema. J Crit Care. 2010;25(3), 508.e1–508.e5085. PubMed
- Krodel DJ, Bittner EA, Abdulnour R, Brown R, Eikermann M. Case scenario: acute postoperative negative pressure pulmonary edema. Anesthesiology. 2010;113(1):200-207. PubMed
- Ma J, Liu T, Wang Q, et al. Negative pressure pulmonary edema (Review). Exp Ther Med. 2023;26(3), 455. PubMed
- Liu R, Wang J, Zhao G, et al. Negative pressure pulmonary edema after general anesthesia: A case report and literature review. Medicine (Baltimore). 2019;98(17), e15389. PubMed
- Luo M, Li M, Qin Z. Negative pressure pulmonary edema resulting from upper airway obstruction during the post-anesthesia recovery period: a case series and literature review. BMC Anesthesiology. 2025;25(1), 125. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.