Copy link
Pharmacogenetics of Anesthetic Agents
Last updated: 10/02/2025
Key Points
- Pharmacogenetics encompasses the relationship between an individual's genetic code and their drug metabolism, exposure, and effects. Genetic variants can also impact drug-drug interactions, which are highly relevant in the field of anesthesia, where the use of polypharmacy is common.
- Early discoveries in this field are integral to the history of Anesthesiology. For example, cholinesterase deficiency, which impacts succinylcholine metabolism, and MH (associated with RYR1 variants) are pharmacogenetic disorders that were discovered over 60 years ago, marking early advances in pharmacogenetics in anesthesia.
- Variations in CYP and UGT enzymes have been associated with altered metabolism of anesthetics, including propofol, volatile agents, benzodiazepines, antiemetics, opioids, and local anesthetics (LA). These pharmacokinetic differences contribute to interpatient variability in anesthetic clearance, exposure, and efficacy. For example, CYP2D6 ultrarapid metabolizers will have reduced antiemetic response to ondansetron, and granisetron (not metabolized by CYP2D6) is a better option.
- Polymorphisms can also influence anesthetic response by altering molecular pathways or drug receptor responses. Examples include MTHFR variants, which increase susceptibility to nitrous oxide neurotoxicity, and MC1R variants in red-haired patients associated with higher anesthetic requirements.
Introduction
- The emergence of the Human Genome Project in the 2000s coincided with a call by the United States Food and Drug Administration for the application of emerging technologies. A shift was made towards improving the efficacy and cost-effectiveness of drug research and design.
- Thus began the era of precision medicine, and the use of genetics to inform personalized care for the right patient at the right time.
- Pharmacogenetics is the study of genetic variations and drug response. Pharmacogenomics refers to the application of genomics in a broader sense (such as gene-gene interactions, gene-environmental interactions, etc.) to drug responses.
- This summary will describe the impact of pharmacogenetics on anesthetic agents categorized by class, highlighting historical perspectives along the way. Please note, some of the basics of pharmacogenetics and their impacts on analgesics are already covered in detail in the OpenAnesthesia summary titled: “Pharmacogenetics of Analgesics.” Link
Overview of Genes
- Table 1 is an overview of genes affecting anesthetic drug pharmacokinetics and dose response. For additional genes relevant to opioid and analgesic pharmacokinetics, see the OpenAnesthesia summary titled “Pharmacogenomics of Analgesics.” Link
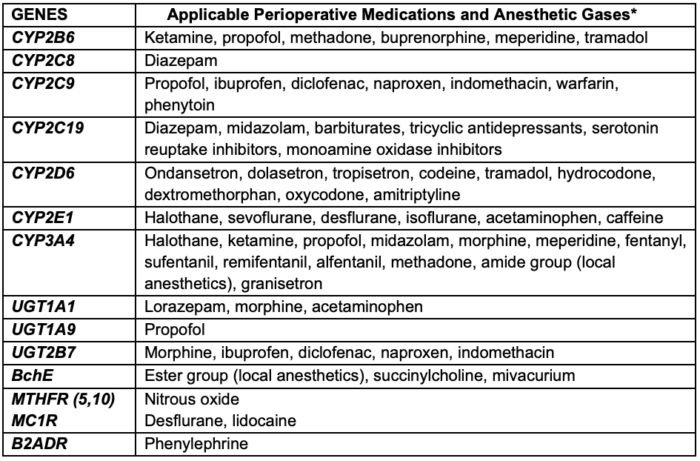
Table 1. Overview of Common Genetic Variants Relevant to Anesthetic Pharmacology. Abbreviations: CYP = Cytochrome P450; UGT = Uridine Glucuronosyltransferase; BChE = Butyrylcholinesterase; MTHFR = Methylenetetrahydrofolate Reductase; MC1R = Melanocortin 1 Receptor; B2ADR = Beta-2 Adrenergic Receptor. *Adapted with permission from Chidambaran V. Genomics relevant to the neuroanesthesiologist. J Neuroanaesthesia Cri Care. 2016;3(4):S44-S52.2
Inhaled Anesthetics
- Inhalational anesthetics act through a site on the gamma-aminobutyric acid type A (GABA-A) receptor.
- Studies exploring the pharmacogenetic properties of sevoflurane and isoflurane have produced results with limited generalizability. In a study of preschool-age children, the AA genotype in the GABAy2 receptor was found to be associated with different anesthetic maintenance requirements and a higher incidence of emergence agitation compared to children with the WT (wild-type) GABAy2 genotype.1
- A study conducted in 20 adult female patients evaluating variations in the Melanocortin 1 Receptor (MC1R) gene, which plays a role in hair pigmentation, found that volunteers with red hair required significantly higher doses of desflurane (mean 6.2 [95% CI, 5.9 – 6.5]) compared to those with dark hair (mean 5.2 [95% CI, 4.9 – 5.5]), P = 0.0004.2
- While subsequent findings have been mixed, the presence of MC1R in neural pathways involved in pain modulation is a proposed mechanism for these effects. More research is needed to fully understand the relationship between MC1R genetics, pain sensitivity, and anesthetic requirements.
Malignant Hyperthermia (MH)
- MH is a pharmacogenetic disorder where susceptible individuals experience a hypermetabolic reaction in skeletal muscle. This condition is triggered by potent volatile anesthetics (excluding nitrous oxide and xenon) and the depolarizing neuromuscular blocker succinylcholine.
- Pathogenic variants in RYR1 (~70% of cases) or CACNA1S (~1%) alter calcium channel regulation, causing sustained intracellular calcium release and uncontrolled muscle contraction.
- Clinical features begin with tachycardia and hypercapnia, followed by rigidity, acidosis, hyperkalemia, hyperthermia, arrhythmia, and cardiovascular collapse.
- The Clinical Pharmacogenomics Implementation Consortium recognizes 50 “diagnostic” variants (48 in RYR1, 2 in CACNA1S) that confer susceptibility; individuals with these genotypes should receive only non-triggering anesthetics.4
- Diagnosis is confirmed by caffeine-halothane contracture testing. Genotype identification enables targeted anesthetic planning, significantly reducing the risk of catastrophic perioperative events.4
Nitrous Oxide
- Nitrous oxide is a nonvolatile odorless gas with low solubility in blood and low potency (high minimum alveolar concentration value). It rapidly diffuses into nitrogen-containing spaces, which may lead to increased pressures within compartments such as the intrathoracic and cranial cavities.
- Nitrous oxide can irreversibly oxidize the cobalt atom of vitamin B12, inhibiting the activity of the enzyme methionine synthase, which converts homocysteine to methionine. Methionine is the principal substrate in several biochemical reactions, including the formation of myelin sheath, neurotransmitters, and DNA synthesis in rapidly proliferating tissues.
- Although the use of nitrous oxide has decreased due to awareness of environmental concerns, due to irreversible inactivation of vitamin B12 (cobalamin), extended intraoperative use of nitrous can lead to acute neuropathies and, rarely, fulminant neurotoxicity.
- The risk is further compounded by the presence of polymorphisms in the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene, resulting in neurological deterioration and death.4
- Single-nucleotide polymorphisms (SNPs) at the C677T and A1298C loci are associated with decreased MTHFR enzymatic activity, leading to the accumulation of homocysteine after nitrous oxide anesthesia, which is linked to higher neurotoxicity.
- Importantly, MTHFR deficiency may go unnoticed in younger pediatric patients, highlighting a situation where obtaining a targeted genotype may prompt a different anesthetic approach.
Propofol
- Propofol exerts its central hypnotic action by activating the GABA-A receptor, which is encoded by the GABRA gene.
- The metabolism of propofol is primarily dependent on hepatic cortical microsomes, with renal tissue contributing to a minor extent. UGT1A9-mediated glucuronidation and the hydroxylation activity of CYP2B6 and CYP2C9 convert the highly lipophilic emulsion into water-soluble metabolites.1
- A schematic overview of propofol genetics at the organ system level is represented below in Figure 1.
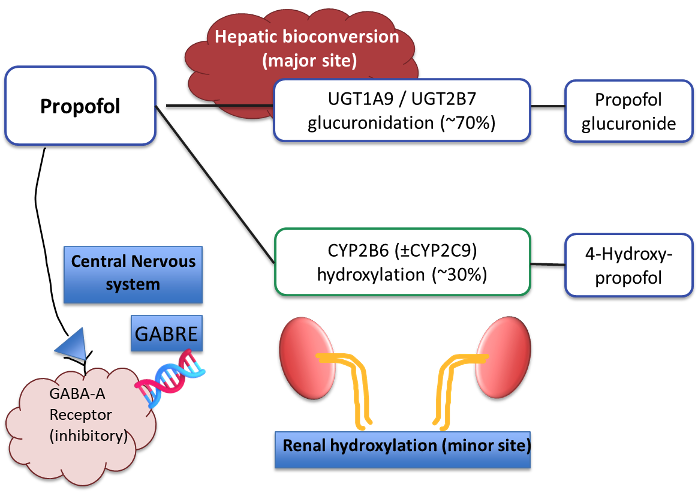
Figure 1. Propofol metabolism and genetics
- The metabolism of propofol is estimated to be 53% dependent on UGT1A9 enzyme activity.
- UGT1A9 gene polymorphisms have yet to demonstrate dose-response changes applicable to clinical practice.
- Widely identified in the literature are isolated differences in propofol clearance linked with CYP2B6 and CYP2C9 polymorphisms; These results, however, have not generated any practical implementation strategies.
- Another research question is; whether there is a relationship between GABA receptor polymorphisms and the relative potency of propofol.
- To date, differences in drug response have not been associated with individual GABRE gene variants.
- Propofol infusion syndrome, a rare but feared complication, is thought to be linked to inborn errors of fatty acid metabolism, although the precise genetic cause and effect relationship has not been studied.
Succinylcholine Metabolism and Pseudocholinesterase Deficiency
- Butyrylcholinesterase (BChE), commonly known as pseudocholinesterase, is synthesized in the liver and is responsible for hydrolyzing the paralytic agents, succinylcholine and mivacurium, as well as ester local anesthetics.
- Succinylcholine combines with the neuronal motor endplate nicotinic acetylcholine receptor to produce depolarization, receptor saturation, and flaccid paralysis.
- Mivacurium is a non-depolarizing neuromuscular blocking agent.
- Knowledge of differential responses to succinylcholine has been present in the medical literature since the 1950s. Today, 30 or more variants of the BChE gene (BChE 3q26.1 -q26.3) are recognized.1
- Deficient pseudocholinesterase activity leads to prolonged apnea after succinylcholine administration. Several factors, including genetic variations, are noted to affect enzyme function.
- Approximately 4% of individuals are heterozygous carriers of atypical alleles, while the majority are homozygous for the typical allele. Homozygous atypical variants are rare but clinically significant (~1 in 3,200-5,000 individuals).6
- Figure 2 dichotomizes individuals based on their BChE enzyme activity, highlighting the clinical effects of known genetic variants.
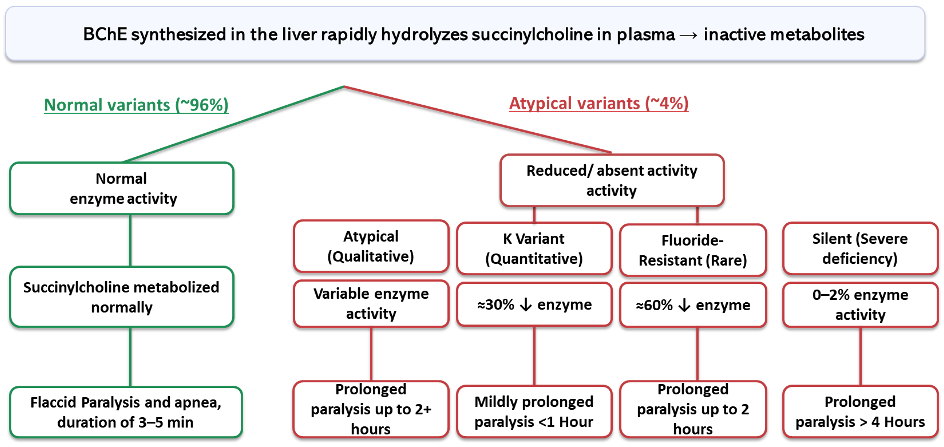
Figure 2. Dichotomy of butyrylcholinesterase enzyme variants for clinical use of succinylcholine. Legend: Green = typical; Red = atypical variants. Data referenced from Am J Med Sci.6
Nondepolarizing Neuromuscular Blockers, Reversal
- Rocuronium bromide competitively binds to postsynaptic acetylcholine receptors at the neuromuscular junction. Rocuronium is excreted in urine (10-25%), and up to 70% is excreted unchanged in bile.7
- Several non-inherited factors are hypothesized to influence the dose-response of rocuronium, including female sex, smoking, and ethnic background.
- A genome-wide association study demonstrated that 4% of the variability in rocuronium dose requirement was explained by variation in the gene SCO1A2. The proposed mechanism involves transporters present in cholangiocytes relevant for biliary rocuronium uptake and excretion.7
- Future studies will help determine the clinical applicability of rocuronium genetic variants, as well as further define the contributions of factors such as age and BMI.
- Agents that reverse neuromuscular blockade, notably neostigmine and sugammadex, are not known to have pharmacogenetic implications.
LA Agents
- Amide and ester LAs act at the level of neuronal fast voltage-gated sodium channels (Na+).
- A feared complication of LA overdosage is LA systemic toxicity, which in severe cases can yield neurotoxicity and cardiovascular collapse.
- In contrast to neuromuscular blockers, the presence of differential pseudocholinesterase activity is of less clinical relevance to ester LAs.
- Commonly used amide LAs, such as lidocaine and bupivacaine, are metabolized primarily by hepatic CYP3A4.
- Ropivacaine is metabolized by CYP1A2, which is not fully developed until 3 years of age. This highlights the increased risk of LA toxicity in children younger than 6 months of age and the need for dosage adjustment.1
- The same group that studied MC1R variation and volatile anesthetic sensitivity also found that red-haired individuals required higher doses of subcutaneous lidocaine than wild type controls.8
- The effects of Na+ channel polymorphisms are a promising area of translational research. Nav1.7 gene variants are being explored in the context of LAs as well as the Brugada syndrome.
Sedative Hypnotics
- Midazolam metabolism is highly dependent on hepatic CYP3A4 and, to a lesser extent, CYP3A5. Despite the identification of numerous CYP3A4 SNPs, there is no consistent association with CYP3A4/5 genetic variation and clinically relevant kinetics.
- Although the heritability of midazolam plasma clearance was estimated to be as high as 96%, studies vary greatly in their genetic estimates. In fact, other factors such as age, sex, co-administered drugs, etc., may play a significant role in variability.
- Studies of CYP2C19 gene variations have identified a gene dosage effect of diazepam metabolism.
- Screening for CYP2C19 variants is available in clinical genotyping assays and can be used to guide diazepam therapy.
- A review of clinically relevant CYP2C19 phenotypes is summarized by the Association for Molecular Pathology (Link).
Dexmedetomidine
- Dexmedetomidine (DEX) is a highly potent α2 adrenergic agonist metabolized by hepatic UGT1A4 and UGT2B10 glucuronidation and CYP2A6 hydroxylation. The ADRA-2A gene sequence encodes the target receptor a-2A.
- Pilot studies have not identified associations of CYP2A6 variants with DEX clearance.
- Associations of ADRA2A gene polymorphisms with the dose-response of DEX-mediated hypotension have been identified.1 The application of these results, as well as other reports of heterogeneity in hemodynamic response, requires further investigation.
Autonomic Receptor Genotypes
- A fundamental role of the anesthesiologist is to modulate sympathetic and parasympathetic tones to maintain end-organ perfusion pressure during the operative process.
- The human B2 adrenergic receptor, with essential roles in bronchial smooth muscle relaxation and peripheral vascular dilation, is encoded by the ADRB2 gene, on chromosome #5.
- Several researchers have observed ethnic variations in the frequency of ADRB2 polymorphisms. Differential hemodynamic responses to laryngoscopy or tracheal intubation and thoracic epidural anesthesia have been observed in patients with known ADRB2 variants.9
- The variability of pressor requirements observed in these two common perioperative scenarios highlights how genotyping may inform the modern clinician’s case preparation.
Postoperative Nausea and Vomiting (PONV)
- Genome-wide association studies on PONV have identified multiple gene variations relevant to the susceptibility of PONV.1
- Given the estimated contribution of PONV to hospital length of stay and perioperative resource expenditure, polymorphisms of CYP2D6, which are responsible for metabolizing the antiemetic 5-HT3 antagonists, are of high relevance.
- CYP2D6 gene duplications are associated with refractory PONV after administration of common 5-HT3 antiemetics such as ondansetron. There are two notable in-class exceptions. Granisetron is metabolized by CYP3A4 primarily, and palonosetron, while metabolized by CYP2D6, induces conformational changes to the 5-HT3A receptor independent of its clearance time.
- Patients have been grouped in translational studies based on their CYP2D6 enzyme activity as Poor metabolizers, Intermediate metabolizers, Extensive metabolizers, or Ultra metabolizers (PM, IM, EM, UM, respectively).10 This information provides practical insight for the genetic considerations of antiemetic agents, and may inform the use of antiemetics tailored to the individual.
References
- Packiasabapathy S, Chidambaran V, Sadhasivam S. Pharmacogenomics. In: C. J. Coté, J. Lerman, B. Anderson, A Practice of Anesthesia for Infants and Children, E-Book; Elsevier; (2024);81-99.e9 Link
- Chidambaran V. Genomics relevant to the neuroanesthesiologist. J Neuroanaesthesia Cri Care. 2016;3(4):S44-S52. Link
- Liem EB, Lin CM, Suleman MI, et al. Anesthetic requirement is increased in redheads. Anesthesiology. 2004;101(2):279-83. PubMed
- Gonsalves SG, Dirksen RT, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for the use of potent volatile anesthetic agents and succinylcholine in the context of RYR1 or CACNA1S genotypes. Clin Pharmacol Ther. 2019;105(6):1338-44. Link
- Selzer RR, Rosenblatt DS, Laxova R, Hogan K. Adverse effect of nitrous oxide in a child with 5,10-methylenetetrahydrofolate reductase deficiency. N Engl J Med. 2003; 349:45-50. PubMed
- Robles A, Michael M, McCallum R. Pseudocholinesterase deficiency: what the proceduralist needs to know. Am J Med Sci. 2019;357(3):263-7. PubMed
- Ahlström S, Bergman P, Jokela R, et al. First genome-wide association study on rocuronium dose requirements shows association with SLCO1A2. Br J Anaesth. 2021;126(5), 949–57. PubMed
- Liem EB, Joiner TV, Tsueda K, Sessler DI. Increased sensitivity to thermal pain and reduced subcutaneous lidocaine efficacy in redheads. Anesthesiology. 2005;102(3):509-14. PubMed
- Frey UH, Karlik J, Herbstreit F, Peters J. β2-Adrenoceptor gene variants affect vasopressor requirements in patients after thoracic epidural anaesthesia. Br J Anaesth. 2014;112(3):477-84. PubMed
- Candiotti KA, Birnbach D, Lubarsky DA, et al. The impact of pharmacogenomics on postoperative nausea and vomiting: Do CYP2D6 allele copy number and polymorphisms affect the success or failure of ondansetron prophylaxis? Anesthesiology, 2005; 102(3), 543–9. PubMed
Other References
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.