Copy link
Phantom Limb Pain
Last updated: 02/08/2024
Key Points
- Phantom limb pain (PLP) is the perception of pain in a limb that has been removed and is a common sequela following an amputation.
- While the exact etiology is still unknown, PLP is thought to occur due to cortical remapping and an irregular interaction between the central and peripheral nervous system.
- Current treatment options include pharmacological agents, mirror therapy, virtual reality, targeted muscle innervation and bionic reconstruction, spinal cord stimulation, etc.
Introduction
- PLP is defined as the perception of pain or discomfort in a limb that is no longer present.1-3 It was first described in the 16th century by Ambroise Paré, a French military surgeon and first recorded by Silas Weir Mitchell, a neurologist in the American Civil War.1-3
- PLP is a common sequela following an amputation and has been reported in up to 50-80% of all amputees. Limb amputations are performed for a variety of reasons, including trauma, diabetes, vascular disease, infection, and cancer.
- It is important to differentiate PLP from residual limb pain (RLP) or “stump” pain which occurs at the actual site of the amputated limb. RLP can result from nerve entrapment, neuroma formation, surgical trauma, ischemia, infection, or skin breakdown. RLP and PLP are commonly associated with each other, but their treatment differs.1
- While classically described after amputation of an arm or leg, PLP can also occur after surgical removal of other body parts, such as the breasts, penis, rectum, testicles, tongue, teeth, eyes, etc.3,4
Clinical Presentation
- The onset of PLP is typically soon after surgery but can be delayed in some patients.
- The pain is often described as stabbing, throbbing, burning, or cramping in nature and tends to be more intense in the most distal portions of the phantom (e.g., foot, toes, hands, fingers).1-3
- The painful episodes are usually intermittent and can last from seconds to minutes to hours. Generally, PLP diminishes in frequency and intensity during the first six months after amputation.
- The pain may be elicited or exacerbated by both physical factors (e.g., weather changes, pressure on the residual limb) and psychological factors (e.g., emotional stress).3
- The nature of the pain often changes with time, such as telescoping, which is the feeling that the phantom limb is gradually shrinking or shortening over time.3,4
Etiology and Mechanisms of PLP
Central Nervous System Changes
- The exact etiology of PLP is still unknown. It was previously postulated that the cause of phantom limb pain is due to cortical reorganization, where an individual’s cortical and peripheral body self-representations remain intact but do not correspond. Neurons that once received input from an area of the body before amputation now respond to new inputs that are nearby the amputated somatosensory region (Figure 1). Further, the lack of visual feedback from the missing limb enhances the mismatch and generates excessive pain, despite the absence of a sensory stimulus.
- Destruction of afferent nerves results in a decrease of neurons releasing gamma-amino butyric acid inhibitory neurons, which normally suppress cortical reorganization.2 The ventral posterior nucleus of the thalamus is reorganized and relays new input into the de-afferented primary somatosensory cortex. Representation of residual limb in the thalamus is enlarged compared to limb in an intact individual.2
- Memory engrams of the limb remain, and certain positional movements in the phantom limb may trigger the pain.
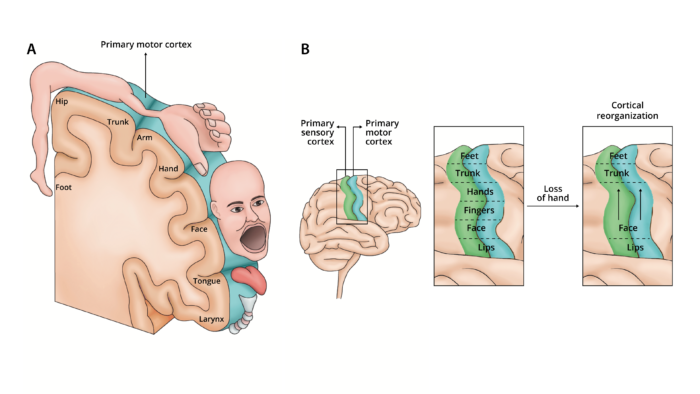
Figure 1. A. Sensory and motor representation from different body parts are laid out in a pattern that forms the cortical homunculus and receives sensory organization (e.g., pain, tactile, olfactory) from different areas of the body. B Cortical reorganization theory. Following an amputation, a cortical region that received sensory or motor projections from the amputated limb to begins to receive input from neighboring cortical regions, which expand to take cover that region that previously controlled the amputated limb. Adapted from Collins KL, et al. A review of current theories and treatments for phantom limb pain. J Clin Invest. 2018;128(6):2168-76. PubMed
Peripheral Nervous System Changes
- Recent studies have focused on peripheral causes of PLP that may contribute to symptoms, focusing on the inability of the severed nerves to repair previous connections, the role of the dorsal root ganglion, and preamputation pain.
- Proximal ends of the dorsal root ganglion (DRG) are disconnected from the spinal cord following amputation causing inflammation and sprouting in the residual limb.2 The injured axons in the peripheral nerves of the residual limb generate spontaneous ectopic loci that propagate along the pathway to the spinal cord (Figure 2).
- DRG hyperactivity occurs due to the upregulation of sodium channels, downregulation of potassium channels, increased expression of neurotrophic factors, and the formation of sympathetic noradrenergic axons into the DRG.
- The spinal cord may become hyperexcitable due to an increase in excitatory peptides released by Aβ fibers following peripheral-nerve injury.3
- The dorsal horn may become more sensitive due to the increased activity of peripheral nociceptors mediated by N-methyl D-aspartic acid (NMDA) receptors and glutamate.4
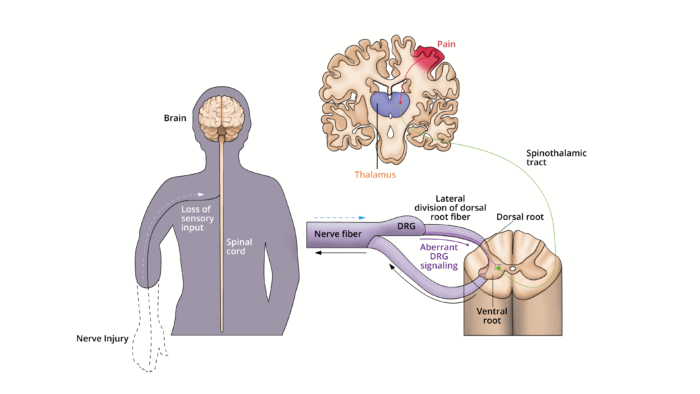
Figure 2. Peripheral nervous system contributions to PLP. The lateral division of the dorsal root fiber from the DRG contain most of the unmyelinated and small myelinated axons that carry pain and temperature information. Following an injury to the nerves, neurons in the DRG increased their nociceptive signaling through increases in neuronal excitability and ectopic discharges. The resulting aberrant signaling through the spinothalamic tract may cause PLP. Adapted from Collins KL, et al. A review of current theories and treatments for phantom limb pain. J Clin Invest. 2018;128(6):2168-76. PubMed
Prevention of PLP
- PLP is seen in most postamputation patients. Thus, it would benefit patients planned for this surgery to obtain prophylactic treatment for PLP.
- Based on the patient’s individual risk and clinical condition, it has been shown that preoperative analgesia, epidurals, and regional blocks could be helpful in attenuating the pain response after surgery. An increasingly popular approach involves placement of perineural catheters either preoperatively or intraoperatively and administration of local anesthetic infusions for 72 hours postoperatively. However, more research is indicated comparing groups who received prophylaxis before and after surgery.4
Pharmacological Options for Treating PLP
- Several medications, including gabapentin, pregabalin, amitriptyline (amongst other tricyclic antidepressants), and ketamine have all been used to reduce the frequency and intensity of neuropathic pain caused by PLP. Unfortunately, most medications are ineffective.5
- Opioids may reduce cortical reorganization in the somatosensory cortex but have a high rate of addiction and dependence.
- Lidocaine has been administered intrathecally or into the foramina to target the dorsal root ganglia. This has temporarily reduced PLP by reducing the hyperexcitability of associated neurons.
Nonpharmacological Options for Treating PLP
- Mirror therapy attempts to restore the missing limb’s projection in the corresponding cortical motor and sensory areas, thus reducing the pain by essentially reconnecting the sensation for motor command and sensory information (Figures 3 and 4).
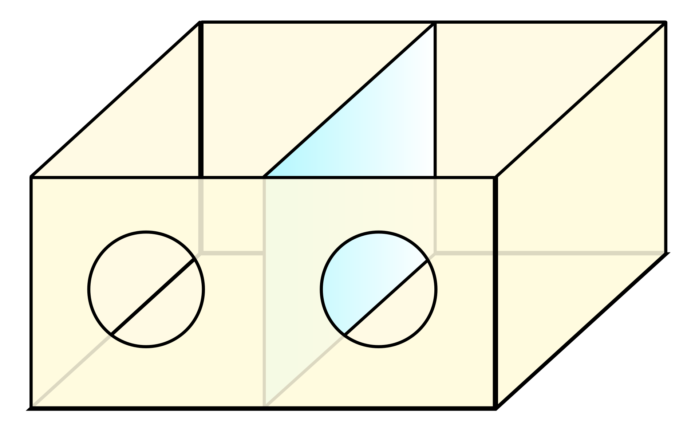
Figure 3. Ramachandran’s mirror box for mirror therapy. The patient inserts a whole hand into one hole and the “phantom” hand into the other. When viewed from an angle, an image of two complete hands is created in the brain. Source: Wikipedia. Link

Figure 4. An occupational therapist using mirror therapy for PLP. Source: Wikimedia. This image was released by the US Navy with the ID 110613-N-YM336-079 Link
- Virtual reality and augmented reality has been as an advanced form of mirror therapy.
- Targeted muscle innervation (TMR) and bionic reconstruction may improve prosthetic control and reduce pain. Reestablishing the anatomic components may reverse the original cortical reorganization that may have caused PLP. Prosthesis coordination with predicated actions via mirror therapy has been shown to be effective in relieving PLP.
- Muscular electrical activity influencing a myoelectric-controlled prosthesis is another promising therapeutic option.
- Other invasive options include spinal cord stimulation and dorsal root ganglion stimulation.
- Cortical stimulation delivering intense peripheral input into the cortical representation area of the amputated limb was found to be effective in reducing chronic pain.4
- Virtual reality (VR) has also shown great promise in allowing patients to use their missing limb in a cost-effective, enjoyable manner. VR may be implemented through a home treatment regimen for patients with PLP.
- Combination therapies with multiple modalities from the above lists may be necessary for patients with refractory PLP by targeting multiple mechanisms involved in PLP.
References
- Hanyu-Deutmeyer AA, Cascella M, Varacallo M. Phantom limb pain. In: StatPearls. Treasure Island (FL): StatPearls Publishing; Accessed September 4th, 2022. Link
- Collins KL, Russell HG, Schumacher PJ, et al. A review of current theories and treatments for phantom limb pain. J Clin Invest. 2018;128(6):2168-76. PubMed
- Flor H. Phantom-limb pain: characteristics, causes, and treatment. Lancet Neurol. 2002;1(3):182-9. PubMed
- Erlenwein J, Diers M, Ernst J, et al. Clinical updates on phantom limb pain. Pain Rep. 2021;6(1): e888. PubMed
- Alviar MJM, Hale T, Lim-Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database of Systematic Reviews 2016; 10. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.