Copy link
Perioperative Patient Blood Management
Last updated: 10/07/2025
Key Points
- Patient blood management (PBM) is a multidisciplinary, evidence-based approach that aims to optimize patient outcomes by preserving a patient’s own blood. It is built on three core pillars: (1) early detection and treatment of anemia, (2) minimizing blood loss and preventing coagulopathy, and (3) maximizing the patient’s physiological tolerance to anemia and coagulopathy. Together, these strategies reduce the need for allogeneic blood transfusions while enhancing oxygen delivery and surgical safety.
- The evolution of PBM from a transfusion-centered approach (focused on the use of blood products) to a patient-centered approach (focused on supporting the patient’s overall blood health) has several advantages, including improved health care delivery, patient outcomes, patient satisfaction, and cost savings.
- PBM strategies span the preoperative, intraoperative, and postoperative periods. These strategies include perioperative anemia screening, cell salvage, retrograde autologous priming, and autologous reinfusion, which reduce reliance on allogenic products, thereby lowering complications and improving patient outcomes.
Introduction
- An expert group representing PBM organizations around the world proposed the following global definition of PBM: “Patient blood management is a patient-centered, systematic, evidence-based approach to improve patient outcomes by managing and preserving a patient’s own blood, while promoting patient safety and empowerment.”1
- PBM aims to optimize blood health with the understanding that blood is an organ and part of a complex, integrated functioning organ system.2 Blood transfusions, though sometimes necessary, should be used judiciously. In contrast to the narrower concept of “optimal blood utilization”, which primarily focuses on the appropriate use of allogenic blood products, PBM takes a broader, patient-centered clinical approach to enhancing overall care, improving outcomes, and reducing complications. Making this distinction helps shift the focus from the product to the patient, sustaining efforts to implement PBM.2
- In 2021, the World Health Organization published a policy brief titled -The Urgent Need to Implement PBM to create awareness about the global burden of anemia and create a sense of urgency to implement PBM.3 “In the past four decades, increased awareness of the inherent risks of transfusion has resulted in major initiatives to mitigate those risks through improvements in blood component safety. The realization that the intense focus on product safety had not been matched with a similar focus on improving transfusion decisions at the bedside led to the concept of “optimal blood use”. The practice of transfusion medicine now emphasizes the judicious use of transfusion, only when clinically indicated. The concept that “our own blood is still the best thing to have in our veins” has given rise to various surgical “blood conservation” techniques (for example, minimization of blood loss, blood salvage, and acute isovolaemic haemodilution).”3
- “Underlying these efforts is the broader concept of ‘patient blood management’ (PBM). This is a patient-centered approach that addresses iron deficiency, anemia, coagulopathy, and blood loss in both surgical and nonsurgical patients as risk factors for adverse medical outcomes. Under PBM, anemia and iron deficiency are recognized as serious global health issues in their own right, affecting billions of people worldwide. Yet, globally, there is still a gap in awareness and implementation of PBM as an overall framework to address the risks of iron deficiency, anemia, blood loss, and coagulopathy.”3
- They further emphasized that PBM is the timely, multidisciplinary application of evidence-based strategies to treat anemia, minimize blood loss, and manage coagulopathy across the continuum of care, while supporting patients during definitive treatment.
- PBM plays a crucial role in modern healthcare by integrating anemia management, minimizing blood loss and coagulopathy, and harnessing tolerance to anemia, with an overarching focus on autologous blood preservation and improved perioperative outcomes.4
- It aims to reduce the reliance on allogeneic blood transfusions, thereby decreasing associated risks such as infections, immunological reactions, and resource constraints.
- Evidence from PBM programs demonstrates that these strategies reduce transfusion rates, shorten hospital stays, and lower costs, without increasing complication rates.
- As an adaptive approach, PBM considers individual patient factors, including comorbidities, genetic predispositions, and religious beliefs, to ensure personalized and ethical care. As healthcare systems strive for high-quality, cost-effective patient care, PBM has become an essential component and topic of discussion.
- Implementation toolkits, benchmarking indicators, or scorecards, and adaptation to pediatric patients should be considered to ensure the successful implementation and sustainability of a PBM program.
- This summary focuses on PBM in patients with pre-existing anemia, coagulopathies, or end-organ comorbidities (i.e., cardiovascular, respiratory, or renal) undergoing surgery or major invasive procedures where significant blood loss and need for transfusion are anticipated (see below).
Common high-risk surgical procedures that may benefit from PBM include:5
- Cardiac and vascular: valve replacement, coronary artery bypass graft, aneurysm repair (open/endovascular), vascular bypass, embolectomy
- Urologic: cystectomy, nephrectomy, prostatectomy
- Orthopedic & spine: femur fracture repair, spinal fusion, amputation
- Abdominal & hepatopancreatic: large tumor excision, pancreatic or liver resection, gastrectomy, splenectomy, bowel/rectal resection
- Gynecologic: myomectomy, total abdominal hysterectomy
Preoperative Considerations: Risk Identification and Optimization
- The preoperative period is the critical window for PBM. Early correction of anemia, standardized risk stratification, and targeted planning to reduce transfusions, complications, and length of stay.
- Mature PBM programs often embed the following steps into preadmission testing clinics or protocols:
Anemia Screening and Correction
- Screen all major surgical candidates for anemia 2–4 weeks before surgery.
- Correct iron deficiency with oral or intravenous iron; consider erythropoiesis-stimulating agents in high-risk patients.
- Replace nutritional cofactors (vitamin B12, folate).
Risk Stratification
- Use standardized bleeding assessment tools and procedure-specific transfusion risk calculators.
- Develop personalized PBM plans with hematology input for patients with sickle cell disease (SCD), hemophilia, thrombocytopenia, or liver disease.
Preoperative Blood Conservation Planning
- Optimize cardiopulmonary and renal comorbidities.
- Discontinue antiplatelet or anticoagulant therapy according to protocol.
- Stop herbal medications that are associated with increased bleeding risk. Please see the OA summary on herbal supplements and anesthesia for more details. (Link)
- Plan for intraoperative conservation techniques [acute normovolemic hemodilution and intraoperative cell salvage].
Laboratory Evaluation
- Complete blood count with reticulocyte index, ferritin, and transferrin saturation.
- Coagulation profile: international normalized ratio (INR), prothrombin time, partial thromboplastin time, fibrinogen.
- Blood type and antibody screen (crossmatch if high risk).
- Obtain informed consent, incorporating religious or cultural considerations.
Special Populations
- SCD: Ensure hydration, maintain oxygenation, and consider transfusion to reduce hemoglobin (Hb) S concentration. See the OA summary on the perioperative management of SCD. (Link)
- Hemophilia and clotting factor deficiencies: Replace deficient factors (factor VIII for hemophilia A, factor IX for hemophilia B) to hemostatic levels; add antifibrinolytics such as tranexamic acid or aminocaproic acid when indicated. See the OA summary on Hemophilia for more details. (Link)
- Liver disease: Correct coagulopathy with fresh frozen plasma (FFP), vitamin K, and/or platelets.
- Leukemia: Anticipate cytopenias; evaluate for disseminated intravascular coagulation; transfuse red cells or platelets, and consult hematology as needed.
Intraoperative Considerations: Minimize Blood Loss and Restrict Transfusion
These include actions taken during surgery to minimize blood loss, optimize oxygen delivery, and reduce the need for allogeneic RBC transfusions.
Massive Hemorrhage Protocols (MHPs)
- In the context of trauma or major hemorrhagic surgical events, rapid recognition and treatment of blood loss is critical to survival.
- The massive transfusion protocol (MTP) is a key component within the broader MHP, focusing on the rapid, ratio-based administration of blood components to ensure optimal blood use. In contrast, the MHP, as part of intraoperative PBM, also addresses coagulopathy, hypothermia, and electrolyte disturbances to support comprehensive hemorrhage management.
- Patients with severe hemorrhage, such as those with trauma (blunt and/or penetrating), surgical complications or injuries from natural disasters, are typically managed with an MHP, which begins with fixed-ratio blood component transfusions (the MTP) and transitions to goal-directed therapy guided by laboratory results and point-of-care testing, including viscoelastic testing (VET) such as ROTEM or TEG.
- Due to time constraints, healthcare providers often administer uncross-matched O-negative blood to MHP patients as an immediate life-saving measure until the patient’s cross-matched blood is identified and becomes available for transfusion.
- The primary objective of MHP is to prevent fatal outcomes due to critical hypoperfusion-related complications.
- Please see the OA summary on massive hemorrhage protocol for more details. (Link)
Indications for MHP Activation and Prediction Tools6
- Blood loss >1500 mL (or 30% of total blood volume)
- Shock index (heart rate [HR]/systolic blood pressure [SBP]) > 1.0, signaling significant blood loss.
- ABC (assessment of blood consumption): score ≥ 2 predicts need for MHP; criteria include penetrating torso injury, SBP < 90, HR > 120, positive focused assessment with sonography in trauma (FAST).
- RABT (resuscitation assessment by transfusion): score ≥ 2 suggests early MHP; based on HR, SBP, mechanism, pelvic fracture, FAST, or transfusion within first hour.
Blood Component Ratios in MTP
- Standard MTP ratio (1:1:1) and (2:1:1) as an alternative4,6
- 1-unit red blood cells (PRBCs)
- 1-unit FFP
- 1-unit apheresis platelets
- Adjunct therapies
- Tranexamic acid
- Calcium supplementation
- Early fibrinogen assessment and replacement (e.g., cryoprecipitate or fibrinogen concentrate)
Goal-Directed Resuscitation
- Recent studies have recommended a restrictive transfusion strategy based on Hb thresholds.4,6
- Hb < 7 g/dL – Stable patients
- Hb < 8 g/dL – Major surgical candidates, acute coronary syndromes, and trauma patients.
- Modern MHP activation emphasizes earlier triggers, such as ≥3 units of RBCs in one hour or loss of >50% blood volume in three hours, to allow for faster, targeted resuscitation. Current best practice also incorporates laboratory and viscoelastic thresholds to guide hemostatic therapy:
- INR >1.5
- Platelets <50 × 10⁹/L
- Fibrinogen <1.5–2.0 g/L
- Please refer to the OA summary on VET for more details. Link
Adverse Reactions to Blood Transfusions
- Acute hemolytic, delayed hemolytic, febrile nonhemolytic, anaphylactic, simple allergic, septic (bacterial contamination), transfusion-related acute lung injury (TRALI), transfusion-associated circulatory overload (TACO)8
- Please see the OA summaries on blood transfusion reactions, TRALI, and TACO (Link) and blood transfusion complications (Link).
Blood Conservation Strategies
- When adjusting for preoperative risks, e.g., in cardiac surgeries, blood transfusions still have an increased risk of morbidity and mortality among exposed patients.7
- Recent blood management guidelines have suggested promising intraoperative blood conservation strategies, including acute normovolemic hemodilution, retrograde autologous priming, and cell salvage.
- Acute Normovolemic Hemodilution (Link)
- It is a technique where a portion of the patient’s blood is withdrawn before or after anesthesia induction and replaced with crystalloid or colloid solutions.
- It reduces blood viscosity and hematocrit, enhancing oxygen delivery and minimizing excessive blood loss during surgery.
- Autologous whole blood can be reinfused intra- or postoperatively, minimizing exposure to allogeneic blood.
- Retrograde Autologous Priming (Link)
- This technique is primarily used in cardiopulmonary bypass surgery, where the patient’s blood replaces the priming fluid in the bypass circuit.
- It reduces the need for crystalloid and colloid administration, thereby reducing the risk of fluid overload and electrolyte imbalance.
- Cell Salvage (Link)
- Blood loss from the patient is collected in a sterile reservoir during surgery. It is then filtered and processed to remove any debris, plasma, free Hb, and contaminants.
- The patient can then be reinfused with the salvaged RBCs.
- Controversy remains for use in cancer surgery or a contaminated surgical field.
- Additional Strategies
- Tolerate anemia with physiologic monitoring (e.g., near-infrared spectroscopy) and mild coagulopathy using VET in conjunction with conventional coagulation testing
- Use antifibrinolytics (e.g., tranexamic acid, aminocaproic acid) and maintain normothermia and acid-base balance
- Employ permissive hypotension when appropriate
- Optimize surgical technique through meticulous hemostasis (e.g., electrocautery, topical hemostatic agents), minimally invasive approaches, experienced assistance, and low blood-draw volume strategies
- Acute Normovolemic Hemodilution (Link)
Postoperative Considerations: Recovery and Blood Health Restoration
The postoperative phase focuses on optimizing recovery while minimizing the need for transfusions. Restrictive transfusion strategies, physiologic monitoring, and early supportive measures improve outcomes and conserve blood resources. Practitioners should consider the following core postoperative PBM strategies:
Monitoring and Decision-Making
- Use restrictive transfusion thresholds: a Hb level of 7–8 g/dL and a hematocrit threshold of 24-27%, unless symptoms or comorbidities justify higher targets.
- Correlate clinical signs (e.g., tachycardia, fatigue, dyspnea) with point-of-care noninvasive Hb and hematocrit monitoring devices.
Optimize Oxygen Delivery
- Provide supplemental oxygen as needed
- Maintain euvolemia and adequate cardiac output
Stimulate Erythropoiesis
- Continue oral or IV iron, folate, and vitamin B12 supplementation
- Consider erythropoiesis-stimulating agents in select patients (e.g., chronic kidney disease, oncology)
Coagulopathy Management
- Continue antifibrinolytic therapy as indicated (e.g., tranexamic acid)
- Use point-of-care VET or hematology consultation to guide therapy
- Monitor postoperative bleeding (e.g., drains, perfusion markers); return to the operating room promptly if hemostasis is required
Minimize Iatrogenic Blood Loss
- Limit phlebotomy; use pediatric collection tubes or reduced draw volumes
- Avoid unnecessary and daily blood sampling
Early Nutrition and Mobilization
- Encourage early oral intake with adequate hydration and nutrition
- Support early mobilization to enhance recovery and stimulate erythropoiesis
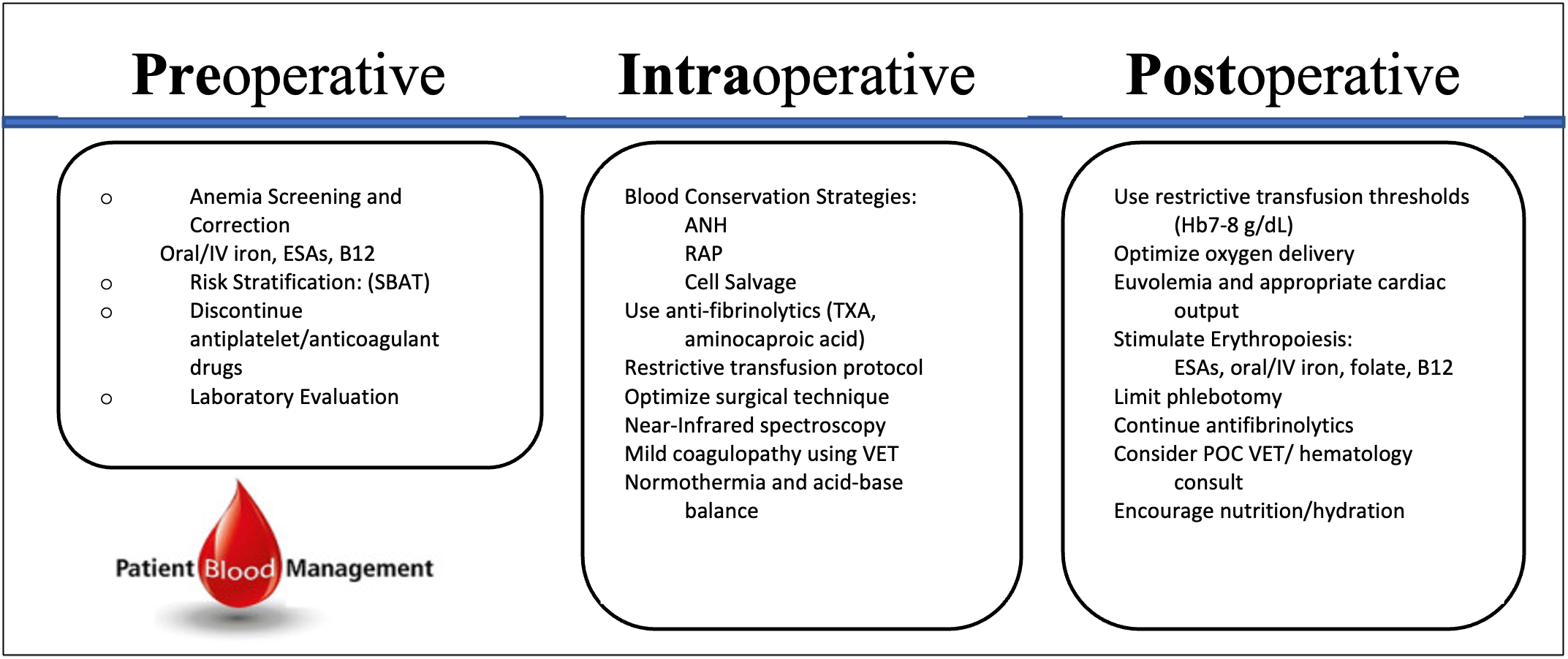
Figure 1. Perioperative PBM Checklist. Outlines key features at each stage of the perioperative patient blood management process, emphasizing critical steps for optimizing patient outcomes. Adapted from Meybohm P, et al. Safety and effectiveness of a Patient Blood Management (PBM) program in surgical patients - the study design for a multi-center prospective epidemiologic non-inferiority trial. BMC Health Serv Res. 2014; 14: 576 (2014). CC BY 4.0 Link
References
- Shander A, Hardy JF, Ozawa S, et al. A global definition of patient blood management. Anesth Analg. 2022; 135(3): 476-88. PubMed
- Ozawa S, Isbister JP, Farmer SL, et al. Blood Health: The Ultimate Aim of Patient Blood Management. Anesth Analg. Published online April 10, 2025. doi:10.1213/ANE.0000000000007528 PubMed
- World Health Organization. The urgent need to implement patient blood management: Policy brief. Accessed Sept 17, 2025. Link
- Franchini M, Marano G, Veropalumbo E, et al. Patient blood management: a revolutionary approach to transfusion medicine. Blood Transfus. 2019;17(3):191-5. Link
- Montroy J, Lavallée LT, Zarychanski R, et al. The top 20 surgical procedures associated with the highest risk for blood transfusion. Br J Surg. 2020;107(13):e642-e643. BJS Link
- Callum JL, George RB, Karkouti K. How I manage major hemorrhage. Blood. 2025;145(20):2245-56. PubMed
- Likosky DS, Dickinson TA, Paugh TA. Blood conservation-A Team Sport. J Extra Corpor Technol. 2016;48(3):99-104. PubMed
- Suddock JT, Crookston KP. Transfusion Reactions. In: StatPearls. Treasure Island, FL. StatPearls Publishing; 2025. Link
Other References
- World Health Organization. The urgent need to implement patient blood management: Policy brief (ISBN: 978-92-4-003574-4). WHO. Created October 19, 2021. Accessed October 7, 2025. Link
- Society for the Advancement of Patient Blood Management (SABM). Administrative and clinical standards for patient blood management programs. Accessed May 15, 2025. Link
- SABM PBM Overview for Clinicians. Module 1 Accessed May 15, 2025. Link
- SABM PBM Overview for Clinicians. Module 2. Accessed May 15, 2025. Link
- Schock S. Pediatric blood management. OA-SPA Pediatric Anesthesia Virtual Grand Rounds. June 2023. Link
- OpenAnesthesia. Herbal Supplements and Anesthesia. Accessed September 16, 2025. Link
- OpenAnesthesia. Hemophilia. Accessed August 9, 2025. Link
- OpenAnesthesia. Sickle Cell Disease: Perioperative Management. Accessed August 9, 2025. Link
- OpenAnesthesia. Massive hemorrhage protocol. Accessed July 6, 2025. Link
- Hartmann J, Hermelin D, Levy JH. Viscoelastic testing: an illustrated review of technology and clinical applications. Res Pract Thromb Haemost. 2022;7(1):100031. PubMed
- OpenAnesthesia. Blood transfusion reactions, TRALI, and TACO. Accessed July 6, 2025. Link
- OpenAnesthesia. Blood transfusion complications. Accessed July 6, 2025. Link
- Barile L, Fominskiy E, Di Tomasso N, et al. Acute Normovolemic Hemodilution Reduces Allogeneic Red Blood Cell Transfusion in Cardiac Surgery: A Systematic Review and Meta-analysis of Randomized Trials. Anesth Analg. 2017;124(3):743-752. PubMed
- Saczkowski R, Bernier PL, Tchervenkov CI, Arellano R. Retrograde autologous priming and allogeneic blood transfusions: a meta-analysis. Interact Cardiovasc Thorac Surg. 2009;8(3):373-376. PubMed
- Frank SM, Sikorski RA, Konig G, et al. Clinical Utility of Autologous Salvaged Blood: a Review. J Gastrointest Surg. 2020;24(2):464-472. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.