Copy link
Perioperative Management of Patients With Type 2 Diabetes Mellitus
Last updated: 07/18/2025
Key Points
- Optimizing patients with diabetes mellitus (DM) for surgery through preoperative planning and education is essential for patient outcomes.
- Intraoperative glucose management can be performed with subcutaneous or intravenous (IV) insulin depending on the clinical scenario.
- Postoperative care is dependent on surgical procedure, patient stability, and nil per os (NPO) status.
Overview of Perioperative Considerations for Diabetic Patients
- There are many facets to the perioperative preparation and management of patients with type 2 DM (T2DM). Essentially, the preoperative assessment focuses on patient optimization and education, intraoperative management emphasizes glycemic control caused by stress-induced hyperglycemia, and postoperative care prioritizes continued hyperglycemia management and return to oral intake.1,2
- This summary will focus on the perioperative management of patients with T2DM. Please see the OpenAnesthesia summary Perioperative Management of Patients with Type 1 DM (T1DM) for special considerations in those patients. Link
- Table 1 displays a summary of perioperative considerations for providers to utilize when evaluating patients with DM.1,2
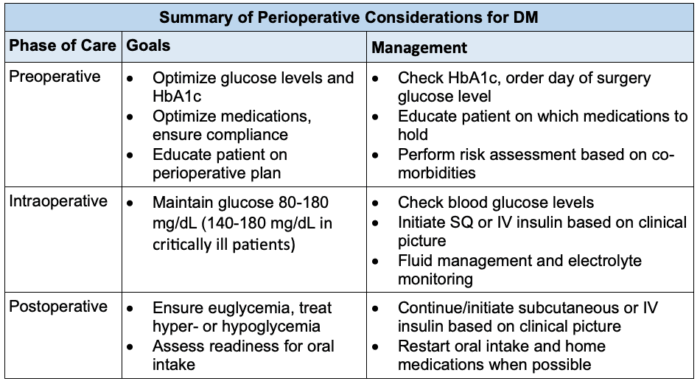
Table 1. Overview of perioperative considerations for DM based on the phase of care.1,2 Abbreviations: DM, diabetes mellitus; HbA1c, glycosylated hemoglobin; SQ, subcutaneous; IV, intravenous
Preoperative Considerations and Planning
- Preoperative assessment should take place with the patient and their support person (if possible) before the day of surgery, preferably in a preanesthesia evaluation clinic staffed by anesthesia providers. If a patient has a complex diabetic history, endocrinology should be involved in developing the patient’s perioperative assessment and plan. The patient’s primary care physician can provide additional insight into the patient’s medical history.1
- During the preanesthesia evaluation, a thorough history regarding the subclassification of DM (e.g., T1DM, T2DM, etc.), duration and severity of the disease process, recent hospitalizations, and current glycemic control and medication adherence should be established.1
Comorbidities and Assessment for Complications1,2
- Identification of preexisting complications (including cardiovascular disease, chronic kidney disease, neuropathy, etc.) likely suggests the presence of moderate to severe disease.
- A thorough review of comorbidities is essential to understand the interplay of the patient’s medical history and disease progression.
Laboratory Values
- Additionally, clinicians should determine the patient’s glycemic control, including HbA1c, within the preceding 3 months, and blood glucose level.
- A target blood glucose of 80-180 mg/dL in the perioperative period is recommended. In critically ill patients, the threshold is narrower at 140-180 mg/dL. A preoperative blood glucose test should be performed prior to surgery.1,3
- If hyper- or hypoglycemia is present, treatment is recommended prior to surgery.1
Medication Review
- The preanesthesia evaluation should include reviewing the patient’s diabetic medication regimen, including oral hypoglycemics and insulin therapy. Patients need to be educated on proper preoperative medication management.1,3
- Please see the OpenAnesthesia summary Glucose Homeostasis and Insulin Therapy (Link) for more details regarding insulin therapy strategies.
Presurgical Antihyperglycemic Medication Management
- Oral and noninsulin injectable antihyperglycemic medications
- It is recommended to stop oral antihyperglycemic and noninsulin injectable medications (e.g., metformin, glucagon-like peptide-1 receptor) agonists, and sulfonylureas) on the day of surgery.1,3
- Sodium-glucose cotransporter-2 inhibitors should be stopped 24 to 72 hours prior to surgery.1
- Home insulin therapy1,4
- Once daily long-acting basal insulin (e.g., glargine, detemir)
- If the long-acting basal insulin dose is taken at lunch or evening time, the dose should be reduced by 20-25% at lunch or evening time the day before surgery (e.g., give 80% of the normal dose).
- If taken in the morning, the normal long-acting basal insulin dose should be administered the day before surgery, and 80% of the normal dose should be administered the morning of surgery.
- Of note, patients on high doses of basal insulin (more than 60% total daily dose (TDD) or insulin TDD > 80 units) or at a high risk of hypoglycemia. Reducing the dose by 50-75% should be considered.
- Twice daily long-acting insulin (e.g., glargine, detemir)
- Patients should decrease the evening dose the day before surgery and the morning dose the day of surgery by 20-25%.
- Intermediate acting insulin (e.g., neutral protamine Hagedorn (NPH))
- No changes are made the day before surgery. The dose should be decreased by 50% the morning of surgery.
- Premixed insulin (e.g., NPH/regular or aspart protamine/aspart)
- No changes are made the day before surgery. The dose should be decreased by 50% the morning of surgery.
- Short-acting insulin
- No changes are made the day before surgery. No morning dose should be administered as the patient should be NPO.
- Subcutaneous (SQ) correctional insulin should be utilized with blood glucose monitoring every 4-6 hours.
- Once daily long-acting basal insulin (e.g., glargine, detemir)
Preoperative Plan
- During the preoperative visit, there should be clear communication and effective education on fasting parameters, preoperative insulin administration, perioperative use of insulin monitors and pumps, and perioperative management of DM.1
Fasting Guidelines
- Standard NPO guidelines are used. The fasting period for patients with DM should be minimized; scheduling these patients for first start or early morning cases should be considered.
Intraoperative Management
- Physiological stress is caused by illness, surgery, and anesthesia, leading to the release of cortisol, glucagon, and catecholamines. Therefore, insulin release and glucose uptake are suppressed, and an increase in insulin resistance, gluconeogenesis and glycogenolysis (Figure 1).1
- Stress-induced hyperglycemia results in osmotic diuresis, including electrolyte imbalance, fluid shifts, ketone production, and proinflammatory marker production. This leads to cellular function damage, immune dysregulation, and poor wound healing.1
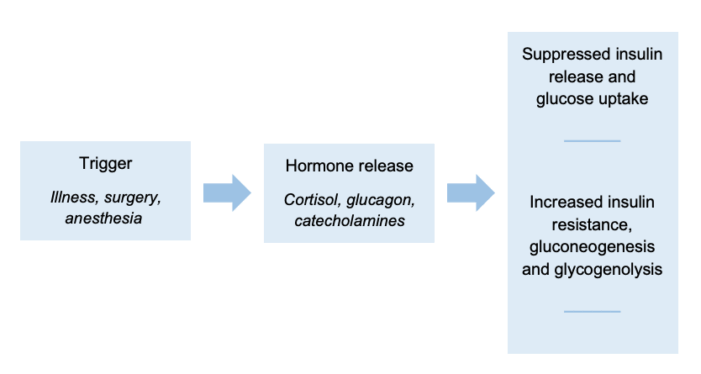
Figure 1. Stress-induced hyperglycemia mechanism chart1
Insulin Management and Glucose Monitoring
- SQ insulin is appropriate for the treatment of hyperglycemia in short (less than 4 hours) cases with limited hemodynamic changes. Frequent glucose checks must be performed. Glucose checks should be performed at least every 2 hours.1,5
- It is important to keep in mind risk factors that may increase a patient’s sensitivity to insulin, including age older than 70 years, glomerular filtration rate less than 45 mL/min, and no prior history of diabetes. Additionally, risk factors that may result in insulin resistance include body mass index greater than 35 kg/m2, home TDD Insulin more than 80 units per day, steroid equivalent of more than 20 mg prednisone daily.6
- IV insulin should be used in long cases, with fluid shifts and hemodynamic instability. Treatment should be initiated at blood glucose levels above 180 mg/dL and checked every 1-2 hours.1,5
- In critically ill patients with a blood glucose level equal to or greater than 180 mg/dL, it is recommended to start an IV insulin infusion. The half-life of insulin is 35 minutes. Glucose monitoring should take place every hour.5
Postoperative Considerations
Postanesthesia Care Unit (PACU) Glycemic Control
- In the PACU, providers need to review intraoperative insulin administration, continue to monitor glucose levels, and treat with subcutaneous or IV insulin as appropriate.
- Patients should be encouraged to resume oral intake of food as soon as clinically appropriate.4 After PACU recovery, stable ambulatory patients may resume normal oral intake and be discharged. They should continue their home antihyperglycemic regimen.1
- For noncritically ill patients who require postoperative hospitalization and are unable to resume oral intake, the suggested insulin regimen is basal insulin (long-acting) and correctional insulin (rapid- or short-acting). For those able to resume normal oral intake, the preferred insulin regimen is basal, nutritional (rapid- or short-acting), and correctional insulin.1
- Critically ill patients should be transferred directly to the intensive care unit (ICU) after surgery. Regular insulin can be used. These patients should be monitored with blood glucose monitoring every 1-2 hours.1
Postoperative Complications
- Elevated glucose levels are associated with poor wound healing, infection, ICU admission, and higher postoperative mortality. Therefore, optimization of DM management and perioperative control of hyperglycemia will benefit patients undergoing surgery.2,6
References
- Dogra P, Anastasopoulou C, Jialal I. Diabetic perioperative management. In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2025. Accessed date: April 16, 2025. Link
- Sapra A, Bhandari P. Diabetes. In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2025. Accessed date: April 16, 2025. Link
- American Diabetes Association. 14. Diabetes care in the hospital: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S144-S151. PubMed
- Crowley K, Scanaill PÓ, Hermanides J, Buggy DJ. Current practice in the perioperative management of patients with diabetes mellitus: a narrative review. Br J Anaesth. 2023;131(2):242-52. PubMed
- Kaur K, Anastasopoulou C, Joyner RW. Diabetes intraoperative management. In: StatPearls (Internet). Treasure Island (FL): StatPearls Publishing; 2025. Accessed date July 7, 2025. Link
- Duggan EW, Carlson K, Umpierrez GE. Perioperative hyperglycemia management: An update [published correction appears in Anesthesiology. 2018 Nov;129(5):1053. Anesthesiology. 2017;126(3):547-60. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.