Copy link
Perioperative Management of Patients with Type 1 Diabetes Mellitus
Last updated: 08/15/2023
Key Points
- Patients with type 1 diabetes mellitus (T1D) require insulin at all times, even while fasting, to maintain euglycemia and prevent ketosis.
- Devices for continuous insulin delivery and glucose monitoring are commonly used. Anesthesiologists must be familiar with their function for perioperative management of patients with T1D.
- The plan for perioperative insulin administration will depend on the type of surgery being performed, metabolic stability of the patient, and potential contraindications to the use of diabetes technology.
Introduction
- Patients with T1D do not secrete endogenous insulin. They require insulin administration at all times, even while fasting, to maintain metabolic stability. Glucose homeostasis, insulin therapy, diabetes devices, and management of hypo- and hyperglycemia are reviewed in a separate OA Summary Glucose Homeostasis and Insulin Therapy.
- This summary provides a review of the perioperative of management of pediatric patients with T1D. The recommendations are based on guidelines published by the International Society for Pediatric and Adolescent Diabetes (ISPAD)1 and the Society for Pediatric Anesthesia (SPA).2
Perioperative Use of Diabetes Devices
- The most recent ISPAD guidelines recommend the use of insulin pump therapy and continuous glucose monitoring for all children with T1D.3,4
- These devices are commonly used by pediatric patients and anesthesiologists are likely to encounter them in the perioperative period. See OA Summary Glucose Homeostasis and Insulin Therapy for a complete explanation of these devices. Please note that some of these devices are very expensive and patients may have limited supplies. If devices are placed incorrectly prior to surgery and then removed perioperatively, this may be extremely costly to the patient.
Perioperative Considerations2
- Contraindications to device use (varies by manufacturer):
- Magnetic resonance imaging (MRI) is an absolute contraindication for insulin pumps and continuous glucose monitors (CGMs). The devices must be completely removed from patients. If the insulin pump has a plastic insertion set, the pump can be removed while leaving the insertion site intact.
- Devices are relatively contraindicated in the setting of ionizing radiation, including computed tomography and nuclear medicine scans, cardiac catheterization, cardiac defibrillator or pacemaker placement, and therapeutic radiation oncology. Devices should be removed in these settings.
- Devices should be used with caution in the setting of x-ray and fluoroscopy. They should be placed out of the direct radiation field and covered with a lead apron. In the setting of electrocautery, it should be placed away from the surgical and ground sites, and the anesthesiologist must confirm that a plastic (not metal) insertion set is being used.
- Devices must be placed outside the surgical field and on a nondependent area of the patient’s body (based on surgical positioning).
CGMs
- CGMs are not validated or FDA-approved for use in perioperative or inpatient settings. Sensor accuracy may be suboptimal in the setting of rapidly changing blood glucose (BG)-values, physical pressure on the sensor, physiologic alterations (including hypothermia, hypotension, vasoconstriction, edema, and hypoxemia), and exposure to certain medications.4,5
- However, there is emerging data supporting CGM use in perioperative and inpatient settings. At this time, the current recommendations support using CGMs to follow perioperative trends, but values should be confirmed with point-of-care (POC) capillary, venous, or arterial samples before making clinical decisions in the perioperative period.2
Insulin Pumps
- Subcutaneous insulin administration is appropriate in the perioperative period for patients who are metabolically stable and undergoing a minor procedure (see Table 1). An insulin pump can be continued for subcutaneous (SC) insulin infusion if there is no contraindication to a pump and the patient or caregiver can confirm:
- that the pump site is intact and has been in place for less than 3 days;
- that the reservoir contains sufficient insulin for the perioperative period; and
- appropriate pump function and current pump settings.
- If the pump will be used intraoperatively, the anesthesiologist must have access to the pump or controller device throughout the perioperative period. Any alarms or signs of metabolic instability may indicate pump failure. In that situation, the pump should be discontinued and replaced with intravenous (IV) insulin infusion.
- If the pump will not be used perioperatively, it should be turned off, physically disconnected from the patient, and given to the caregiver. An IV insulin infusion should be started within 30 minutes of disconnecting the pump to provide appropriate basal insulin coverage.
Closed Loop Insulin Delivery Systems
- Closed-loop insulin delivery systems rely on CGM readings to adjust insulin delivery. Because CGM values are not validated for treatment decisions in the perioperative period, closed-loop insulin delivery should not be used perioperatively.1
- It is not always immediately obvious when closed-loop insulin delivery is active, so this should be specifically addressed preoperatively with the patient or caregiver for patients who use both an insulin pump and CGM. If closed-loop delivery is active, the patient or caregiver must disable it and resume a fixed basal rate (manual mode) prior to going to the operating room. If the patient or caregiver does not know how to do this, the insulin pump should be discontinued perioperatively.
Preoperative Assessment and Planning1,2
- Preanesthesia evaluation and care coordination with the proceduralist and patient or caregiver should occur prior to the day of the procedure. When possible, the patient’s primary endocrinologist should be consulted for an individualized management plan, particularly for patients using neutral protamine Hagedorn (NPH) insulin and those at risk for fasting hypoglycemia. Instructions for fasting, preoperative insulin administration, and perioperative use of diabetes technology should be clearly communicated to patients and caregivers, particularly for those who are newly diagnosed and inexperienced with managing insulin administration while fasting.
Patient Assessment
- Assess glycemic control: The patient’s hemoglobin A1c (HbA1c), typical BG values, and frequency hypoglycemia and diabetic ketoacidosis (DKA) should be determined. Patients with poor glycemic control should have elective procedures postponed if the delay will allow improved glycemic control preoperatively. There is no consensus on an appropriate HbA1c target.
- Document the insulin regimen: The patient’s home regimen, including the method of insulin administration (multiple daily injections vs. continuous SC insulin infusion), type of insulin administered, insulin dosing parameters (basal rate, insulin-to-carbohydrate ratio [ICR], and correction factor [CF]), and the use of diabetes technology (insulin pumps, CGMs) should be determined. See OA Summary Glucose Homeostasis and Insulin Therapy for a complete explanation of these terms.
Scheduling Considerations
- The patient should be scheduled as the first case of the day whenever possible to minimize fasting time. Patients with poorly controlled diabetes or frequent fasting hypoglycemia may require preoperative admission for IV dextrose and insulin while fasting.
Communication with the Patient or Caregiver
- Fasting: The patient should follow standard perioperative fasting guidelines with anticipatory guidance for the management of hypoglycemia. Patients may drink carbohydrate-containing clear liquids until 2 hours before surgery and should be encouraged to do so if they have a history of fasting hypoglycemia. If they become hypoglycemic prior to arrival in preoperative holding, oral glucose liquids, tablets, or gels should be administered and disclosed to the anesthesiologist upon arrival. Tablets and gels may or may not be considered fasting violations depending on institutional guidelines.
- Insulin administration:
- Patients should continue their normal basal insulin administration preoperatively. This includes long-acting SC insulin boluses as well as the basal infusion on an insulin pump. Those with a history of fasting hypoglycemia may need a 20-30% reduction in their basal dose to maintain euglycemia, but it should not be held.
- No rapid- or short-acting insulin boluses should be administered while the patient is fasting unless BG is >250 mg/dL.
- Diabetes technology: If the patient uses an insulin pump or CGM, they should be instructed on proper device placement and duration of wear (see section on insulin pumps and CGM above).
- Evaluation on arrival to preoperative holding:
- The patient’s last oral intake, current BG, home insulin regimen, and last insulin administration (basal and bolus) should be verified. If the patient is hypo- or hyperglycemic, see management recommendations below.
Perioperative Glucose Management
Glucose Goals
- Perioperative dysglycemia is associated with adverse clinical outcomes.6 However, intensive insulin therapy is associated with an increased rate of hypoglycemia without improvement in clinical outcomes.7
- Pediatric patients, and especially young children, are at higher risk for hypoglycemia than adult patients.6,8 Given this data, the ISPAD recommends a perioperative BG range of 90-180 mg/dL for children with diabetes.1 However, correction doses of insulin are not recommended perioperatively unless the BG is >250 mg/dL to minimize the risk of hypoglycemia while fasting.1,2
Glucose Monitoring
- Patients receiving insulin in the perioperative period should have BG checked at least hourly. BG should be checked every 30 minutes if there is a change in insulin or dextrose administration and every 15 minutes if hypoglycemia occurs.2
- CGMs can be used to follow perioperative trends, but values should be confirmed with POC capillary, venous, or arterial samples before making clinical decisions in the perioperative period.2 Insulin requirements may be altered in the perioperative period due to changes in oral intake, nausea, stress, pain, and inactivity. Therefore, it is important to monitor BG closely for 24-48 hours after procedures.1
Glucose Administration
- Patients who are euglycemic and undergoing minor procedures with SC insulin administration may not require dextrose administration. However, IV dextrose is required for patients undergoing major procedures, those who are hypoglycemic, and those who are at risk for hypoglycemia (e.g., frequent fasting hypoglycemia episodes or maintained on NPH for basal insulin).
- Five percent dextrose infusions are generally sufficient. However, 10% dextrose may be used for patients at high risk of hypoglycemia or those with an initial BG less than 100 mg/dL.2
Perioperative Insulin Administration
- Patients with T1D require basal insulin administration, even while fasting. They may require additional correction doses of insulin if their BG is not within the recommended perioperative range (90-180 mg/dL). Insulin can be administered via SC or IV routes. The route of insulin administration depends on the type of surgery, metabolic status of the patient, and the ability to continue an insulin pump use perioperatively.1,2
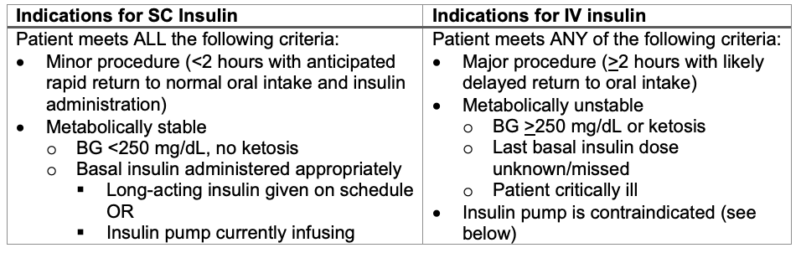
Table 1. Indications for SC and IV insulin
Perioperative SC Insulin Administration
- Basal insulin coverage is provided by the patient’s most recent long-acting insulin injection or the basal rate on their insulin pump.
- Bolus insulin
- Rapid- or short-acting insulin boluses should not be administered while the patient is fasting unless the patient’s BG is >250mg/dL. Use the patient’s CF to calculate the amount of insulin required to decrease BG to 150 mg/dL. Correction doses of insulin should not be given more often than every 3 hours to minimize dose stacking and subsequent hypoglycemia.
- Once the patient has resumed oral intake postoperatively, use the patient’s ICR to calculate the appropriate amount of insulin to cover their carbohydrate intake.
Perioperative IV Insulin Administration
- IV insulin can be started at the patient’s basal infusion rate. Caution is required when converting from SC to IV insulin dosing. SC insulin is NOT weight-based, while IV insulin is weight-based (units/kg/hour).
- If the patient’s basal rate is unknown, the following chart can be used (Table 2).1 Note that this chart is based on dosing regimens for patients who are critically ill or experiencing DKA. It may overestimate the insulin needs of surgical patients who are otherwise well-controlled and metabolically stable.
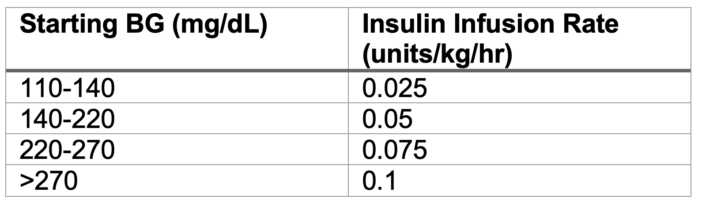
Table 2. Dosing regimen for insulin infusion
- The infusion rate should be titrated by 0.01-0.03 units/kg/hr to maintain euglycemia. Boluses should NOT be administered.
- IV insulin and dextrose should always be coadministered through the same IV line.
- Due to the short half-life of IV insulin (5 minutes), SC insulin should be administered at least 15 minutes before stopping the IV infusion.
Postoperative Care2
Criteria to Discharge Home
- Patients should demonstrate tolerance of oral intake with a transition to their home insulin regimen prior to discharge home. The patient’s ICR should be used to calculate the short-acting insulin bolus required to cover their oral intake.
- For patients using an insulin pump, appropriate pump placement and function should be verified with the patient or caregiver.
- Patients should be advised to closely monitor their BG levels for 24-48 hours postoperatively, as their insulin requirements may be altered by nausea, stress, pain, and/or inactivity.1
Patients Requirement Inpatient Care
- Persistent hypo- or hyperglycemia, ketosis, or intolerance of oral intake are criteria for postoperative admission. For any patient being admitted, information about their metabolic status, including last BG and insulin administration, should be relayed directly to the admitting team.
Complications from Insulin Therapy
Hypoglycemia
- Excess insulin administration results in hypoglycemia. Hypoglycemia should be treated when BG is less than 70 mg/dL or if the patient is symptomatic9 (Figure 1). The priority for treatment should be administering additional glucose, rather than holding insulin, because of the risk of ketosis if basal insulin is held for a prolonged time. If glucose cannot be administered, BG is less than 60 mg/dL, or the patient has neurologic impairment, glucagon should be administered and/or insulin should be held.
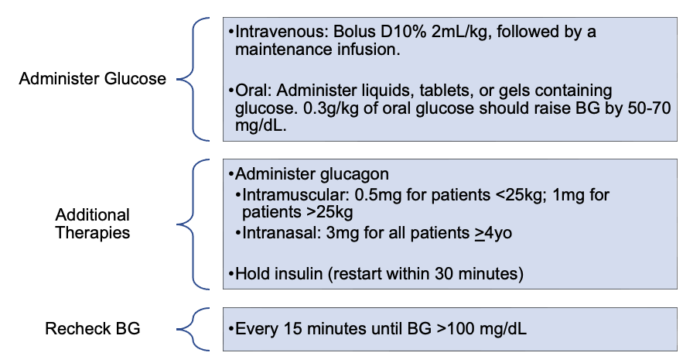
Figure 1. Management of hypoglycemia2,9
Hyperglycemia/DKA
- Insufficient insulin administration results in hyperglycemia and, potentially, DKA. Insulin should be administered to return BG to the patient’s target level (Figure 2). In the perioperative period, BG should be maintained within a target range of 90-180mg/dL. In addition, any BG >250 mg/dL should trigger an evaluation for DKA.2 Treatment protocols for DKA are complex and outside the scope of this document.
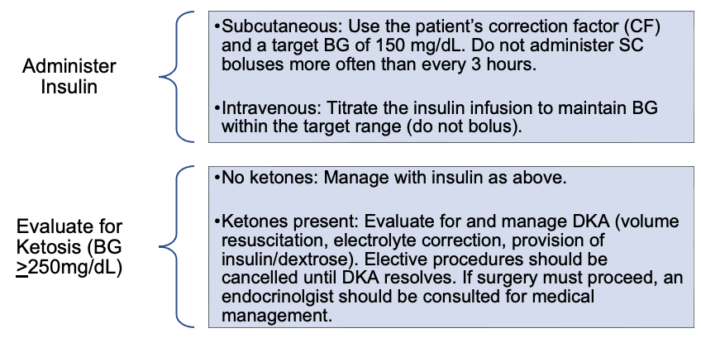
Figure 2. Management of hyperglycemia2,10
Insulin Pump Malfunction2
- Any unexplained metabolic instability (dysglycemia or ketosis) or pump alarms should raise concern for pump malfunction. In the event of pump malfunction, the pump should be turned off and physically disconnected from the patient. An IV insulin infusion should be started.
References
- Kapellen T, Agwu J, Martin L, et al. ISPAD clinical practice consensus guidelines 2022: Management of children and adolescents with diabetes requiring surgery. Pediatr Diabetes. 2022;23(8):1468-77. PubMed
- Martin LD, Hoagland MA, Rhodes ET, et al. Perioperative management of pediatric patients with type 1 diabetes mellitus. Updated recommendations for anesthesiologists. Anesth Analg. 2020;130(4):821-7. PubMed
- Sherr JL, Schoeler M, Dos Santos TJ, et al. ISPAD clinical practice consensus guidelines 2022: Diabetes technologies: Insulin delivery. Pediatr Diabetes. 2022;23(8):1406-31. PubMed
- Tauschmann M, Forlenza G, Hood K, et al. ISPAD clinical practice consensus guidelines 2022: Diabetes technologies: Glucose monitoring. Pediatr Diabetes. 2022;23(8):1390–1405. PubMed
- Buschur EO, Faulds E, Dungan K. CGM in the hospital: Is it ready for prime time? Current Diabetes Reports. 2022;22(9):451-60. PubMed
- Vanderhoek SM, Prichett L, Hardeo H, et al. Association of dysglycemia with post-operative outcomes in pediatric surgery. J Pediatr Surg. 2023;58(3): 365-72. PubMed
- Buchleitner AM, Martinez-Alonso M, Hernandez M, et al. Perioperative glycaemic control for diabetic patients undergoing surgery. Cochrane Database Syst Rev. 2012;12(9):CK007315. PubMed
- Hirshberg E, Larsen G, van Duker H. Alterations in glucose homeostasis in the pediatric intensive care unit: Hyperglycemia and glucose variability are associated with increased mortality and morbidity. Pediatr Crit Care Med. 2008;9(4):361-6. PubMed
- Abraham MB, Karges B, Dovc K, et al. ISPAD clinical practice consensus guidelines 2022: Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2022;23(8):1322-40. PubMed
- Glaser N, Fritsch M, Priyambada L, et al. ISPAD clinical practice consensus guidelines 2022: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2022;23(7):835-56. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.