Copy link
Pediatric Myocarditis
Last updated: 11/17/2023
Key Points
- Myocarditis is an inflammatory disease of the myocardium.
- The clinical spectrum ranges from mild, nonspecific symptoms to malignant arrhythmias and acute or chronic heart failure.
- The anesthetic management goals for patients with myocarditis are similar to those for patients with dilated cardiomyopathy and heart failure.
Introduction
- Myocarditis is an inflammatory disease of the myocardium which results in myocardial dysfunction and a spectrum of related sequela.
- The incidence of myocarditis is difficult to determine, as mild or subclinical presentations often remain undiagnosed. Myocarditis accounts for 0.05% of pediatric admissions, with a relatively higher incidence of mortality compared to other diagnoses.1 In autopsy studies of pediatric patients, biopsy-proven myocarditis accounted for 2% of infant deaths, and 5% of deaths in children over age 5.1
- Myocarditis is more common and is associated with poorer prognosis in children younger than age 2.1,2 In pediatric dilated cardiomyopathy (DCM) of known cause, up to 46% are due to myocarditis.1
Myocarditis
Etiology
Although the etiology remains undetermined in many patients, myocarditis may be caused by multiple etiologies which are often classified as infectious or noninfectious.
Infectious1,2
- Viral infection is most prevalent cause of myocarditis in developed countries.
- There are nearly 20 cardiotropic viruses.
- Historically, adenovirus and enteroviruses (Coxsackie group B) were most common.
- Currently, myocarditis is primarily due to parvovirus B19, human herpes virus 6, and adenovirus.
- Most recently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been identified as a cause of myocarditis.
- Bacterial infection1
- Lyme disease (Borrelia burgdorferi) can cause acute and chronic myocarditis, but is typically associated with full recovery.
- Diphtheria can also cause myocarditis.
- Protozoa1
- Chaga’s disease (Trypanosoma cruzi) is common in South and Central America. It is characterized by pericardial effusion in the acute phase, and by left ventricular apical aneurysms in the chronic phase.
Noninfectious1,2
- Autoimmune-mediated myocarditis is characterized by systemic inflammation and often includes both myocarditis and pericarditis. Example etiologies include rheumatic fever, Kawasaki disease, lupus, or scleroderma.
- Hypersensitivity myocarditis is characterized by peripheral eosinophilia or eosinophilic infiltrates on myocardial biopsy. Medication-induced hypersensitivity is commonly caused by antibiotics, central nervous system agents, and a variety of others. Other causes include hypereosinophilia syndromes and parasitic infection.
- Toxic myocarditis is due to the direct effects of medication or other toxic agents such as illicit drugs, chemicals such as arsenic, or insect venom.
- Postvaccination myocarditis has been reported 1-4 days following administration of mRNA COVID-19 vaccines. The pathophysiology underlying this rare complication is unknown; however, most patients recover within 10 days with no residual dysfunction by echocardiography.3
Pathophysiology
The pathophysiology of myocarditis can be divided into three overlapping phases.1,2
Acute Myocardial Injury
- The virus enters cardiac cells (cardiomyocytes, endothelial cells, and stromal cells) via pathogen-specific receptors. For example, the coxsackie and adenovirus receptor is expressed on cardiomyocytes with peak expression in the perinatal period, explaining why there is increased neonatal susceptibility and risk for fulminant infection.1
- Viral replication leads to myocyte necrosis.
Immunologic Response2
- Innate: Myocardial necrosis leads to innate immune cell activation via pattern recognition receptors, acute inflammatory mediators, and nitric oxide release. This leads to neutrophil and monocyte production. Monocyte infiltration causes further damage.
- Adaptive: Antigen-specific T and B cells are produced several days after infection. Although CD8+ T cells are required for viral clearance, CD4+ T cells can further contribute to pathogenesis. Interleukin 17A can cause progression to cardiac fibrosis and DCM due to fibroblast activation and further monocyte infiltration.
- Autoimmune: Evidence of autoimmune response is present in some cases, and is suggested by presence of autoantibodies, coexisting autoimmune disease, familial clustering, or HLA-DR4.
Chronic Phase
- Myocardial injury and inflammation may resolve with full recovery.
- Ventricular remodeling with DCM may occur, with or without persistence of viral and inflammatory response.
Clinical Presentation & Diagnosis
- Myocarditis presents with a wide range of clinical profiles.1,2,4 A clinical diagnosis is based on a constellation of supportive findings. The absence of individual examination or investigative findings does not preclude diagnosis. A recent infectious illness or viral prodrome is common.
- Clinical presentation may include mild nonspecific symptoms (fatigue, dyspnea, fever, nausea, abdominal pain, poor feeding) often resembling noncardiac related illness, acute heart failure without cardiogenic shock, fulminant myocarditis with cardiogenic shock, arrhythmias, sudden death without other symptoms, myopericarditis with acute coronary syndrome symptoms, and or chronic, gradual progression of heart failure symptoms.
Lab Findings1,2,4
- Inflammatory biomarkers: elevated C-reactive protein, erythrocyte sedimentation rate (low negative predictive value)
- Markers of myocardial injury: elevated Troponin T (specificity 86%, sensitivity 71%), Troponin I, or creatine kinase muscle-brain
- B-type natriuretic peptide may be elevated secondary to ventricular dilation.
- Viral serology: Unclear diagnostic value, may not correlate with biopsy results
Electrocardiogram1,2,4
- Variable, nonspecific ST-T wave changes, ST-segment elevation mimicking ischemia
- Low-voltage QRS complexes, particularly in limb leads
- Atrioventricular conduction delays
- New-onset 3rd degree AV block (requires rule out of myocarditis in pediatric patients)
- Tachyarrhythmias
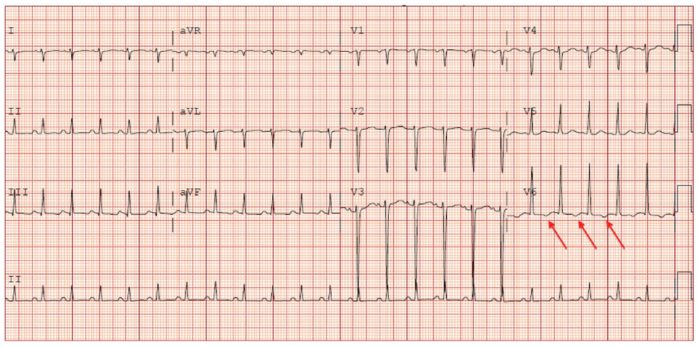
Figure 1. T wave inversion in the lateral leads of a 12‐year‐old female patient presenting with myocarditis. T wave inversion in lateral leads is a sign of left ventricular strain which may be indicative of myocarditis. Used with permission from Dasgupta S, et al. Myocarditis in the pediatric population: A review. Congenit Heart Dis. 2019;14(5):868-877. PubMed
Echocardiography4
- Mild to severe changes in left ventricular (LV) function, segmental wall motion abnormality, thickened myocardium, LV dilation. Right ventricular dysfunction may occur. Fulminant myocarditis may have preserved LV chamber size, with diminished function and wall thickening.
- Pericardial effusion may indicate pericardial involvement.
- Decreased longitudinal strain patterns may exist even in setting of preserved LV function, and correlate with tissue pathology or other cardiac MRI findings.
Cardiac Magnetic Resonance Imaging (CMRI)
- In children, CMRI can identify myocardial injury and differentiate myocarditis from noninflammatory cardiomyopathy.4
- Noninvasive gold standard, only second to endomyocardial biopsy.
- T2-weighted imaging evaluates myocardial edema.2
- T1-weighted imaging with gadolinium2
- Early enhancement – allows for assessment of hyperemia
- Late enhancement – suggestive of myocardial fibrosis
- Modified Lake Louise Criteria is used as a diagnostic tool based on findings above.
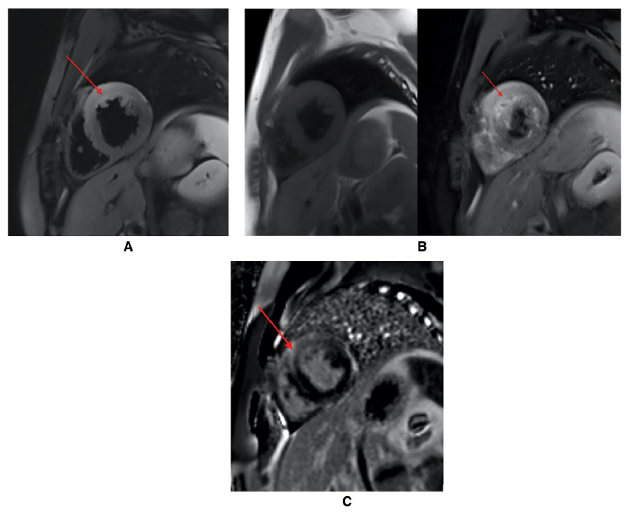
Figure 2. (A). T2 weighted cMRI demonstrating edema (red arrow) in a 15‐year‐old male patient with myocarditis. (B). T1 weighted cMRI (precontrast and postcontrast) demonstrating hyperemia (red arrow) in a 15‐year-old male patient with myocarditis. (C). Late gadolinium enhancement demonstrating fibrosis (red arrow) in a 15‐year‐old male patient with myocarditis. Abbreviation: CMRI, cardiac magnetic resonance imaging Used with permission from Dasgupta S, et al. Myocarditis in the pediatric population: A review. Congenit Heart Dis. 2019;14(5):868-877. PubMed.
Endomyocardial Biopsy
- Gold standard for diagnosis, defined by Dallas criteria for histopathologic classification.1
- Recommended for patients with:
- Acute (< 2 weeks) heart failure with hemodynamic compromise +/- LV dilation2
- Subacute heart failure (2 weeks to 3 months) with LV dilation, ventricular arrhythmias, or high-grade block2
- Symptoms unresponsive to treatment within 1-2 weeks2
- Inflammatory cellular infiltrate with myocyte necrosis not characteristic of ischemia1
- High false negative error can occur due to focal, patchy myocardial infiltrates and random biopsy location with inability to biopsy LV free wall (predominant pathologic location).4
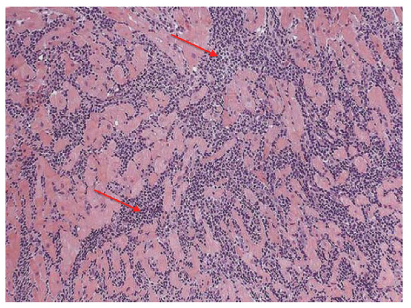
Figure 3. An endomyocardial biopsy specimen of a 15‐year-old male patient with myocarditis. This biopsy specimen demonstrates diffuse neutrophilic infiltration (red arrows) indicative of myocardial inflammation. Used with permission from Dasgupta S, et al. Myocarditis in the pediatric population: A review. Congenit Heart Dis. 2019;14(5):868-877. PubMed
Treatment
- Anticipatory and supportive critical care is based on cardiovascular status and risk of hemodynamic compromise. This may include:
- Hemodynamic support – Inotropes (milrinone, dopamine, epinephrine)
- Mechanical ventilation
- Mechanical circulatory support (MCS) may be required. Registry data shows 23% of hospitalized patients in early phase require MCS.4
- Long-term MCS and severe chronic heart failure may require cardiac transplant.
- Patients with persistent ventricular dysfunction may require heart failure management including angiotensin-converting enzyme inhibitor or angiotensin receptor antagonists, beta-adrenergic blockers, and/or spironolactone.
- Arrhythmias may require pharmacologic treatment, temporary or permanent pacing, and/or an implantable cardioverter-defibrillator.
- Immunosuppressive agents are commonly used in acutely decompensated patients and may also benefit those with autoimmune myocarditis.2,4
- Intravenous immunoglobulin, corticosteroids, and antiviral therapy have conflicting evidence and are variably used among pediatric centers.2,4
Long-Term Outcome
- Acute myocarditis with normal cardiac function is associated with a good prognosis and high likelihood of spontaneous recovery.1
- In fulminant myocarditis with adequate supportive treatment, recovery is also likely.1
- Giant-cell myocarditis carries a poor prognosis for children and adults with median survival less than 6 months without heart transplant.1
- Hypotension, elevated troponin I or brain natriuretic peptide, and decreased ejection fraction increase mortality risk in children.1
- Arrhythmias may be associated with poor early outcome.4
- Many children will recover functional capacity. Exercise should be limited for first 6 months due to risk of sudden death, regardless of severity of myocarditis.4
- Patients should follow up with cardiology regarding ongoing monitoring and discontinuation of heart failure medications, as slow remodeling can occur overtime leading to DCM.4
Anesthesia Considerations5
- Anesthetic management goals are similar to those for dilated DCM and heart failure.
- Preoperative assessment should focus on the evaluation of ventricular function, optimization of volume status, correction of electrolyte abnormalities, and management of arrhythmias.
- The hemodynamic goal for induction and maintenance of anesthesia is to maintain myocardial and end-organ perfusion and oxygen delivery:
- Maintain sinus rhythm and normal heart rate
- Avoid increases in afterload
- Maintain preload given elevated left ventricular end diastolic pressure
- Maintain contractility and avoid/limit myocardial depressant medications
- Maintain normal hemoglobin and oxygen saturation
- Inotropic and/or vasoactive support may be required to support myocardial function or normalize systemic vascular resistance.
- Invasive pressure monitoring and/or transesophageal echocardiography should be considered based on the severity of myocardial dysfunction and the nature of the surgical procedure.
- For patients who require mechanical circulatory support, left ventricular unloading is important and may require an atrial septostomy or a surgically place vent.
References
- Tunuguntla H, Jeewa A, Denfield SW. Acute myocarditis and pericarditis in children. Pediatr Rev. 2019;40(1):14-25. PubMed
- Dasgupta S, Iannucci G, Mao C, et al. Myocarditis in the pediatric population: A review. Congenit Heart Dis. 2019;14(5):868-77. PubMed
- Sandeep N, Fairchok MP, Hasbani K. Myocarditis after COVID-19 vaccination in pediatrics: A proposed pathway for triage and treatment. J Am Heart Assoc. 2022;11(21):e026097. PubMed
- Law YM, Lal AK, Chen S, et al. American Heart Association pediatric heart failure and transplantation committee of the Council on Lifelong Congenital Heart Disease and Heart Health in the Young and Stroke Council. Diagnosis and management of myocarditis in children: A scientific statement from the American Heart Association. Circulation. 2021 10;144(6): e123-e135. PubMed
- Davies MR, Cousins J. Cardiomyopathy and anaesthesia, Continuing Education in Anaesthesia Critical Care & Pain. 2009; 9(6):189–193. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.