Copy link
Pediatric Cardiomyopathy
Last updated: 05/24/2023
Key Points
- Cardiomyopathy (CM) is a disease of the myocardium that poses a significant risk of perioperative morbidity, including arrhythmia, myocardial ischemia, cardiac arrest, and even death. Being asymptomatic does not imply benign disease.1,2
- Providing safe anesthesia to children with CM requires an understanding of the pathophysiology and careful evaluation of cardiac conditions.
- Anesthetic strategies can vary among different types of CM, but the overall goal is always to maintain cardiac output.
Introduction
- CM is a disease of the myocardium associated with cardiac dysfunction.2 Despite a low incidence in the pediatric population, it poses a significant risk of perioperative morbidities, such as arrhythmia, myocardial infarction, and cardiac arrest.
- The overall incidence of pediatric CM is 1-1.5 cases per 100,000 patients.
- HCM is the most common cause of sudden cardiac arrest in young people.1,2
- CM is the primary indication for heart transplantation in children.
- Pediatric CM can be categorized into 5 subtypes based on etiology and pathophysiology:2
1. Dilated cardiomyopathy (DCM)
2. Hypertrophic cardiomyopathy (HCM)
3. Restrictive cardiomyopathy (RCM)
4. Noncompaction cardiomyopathy (NCM)
5. Arrhythmogenic right ventricular dysplasia (ARVD) - The three most common phenotypes are DCM (60%), HCM (25%), and NCM (9%).2,3
- Associated syndromes/comorbidities:3
- Syndromes: Noonan, Costello, cardiofaciocutaneous syndromes
- Metabolic disorders: Pompe, Fabry, Hunter/Hurler, Barth syndromes
- Neuromuscular disorders: Duchenne/Becker muscular dystrophy, limb-girdle muscular dystrophy, Friedreich ataxia, myofibrillar myopathy
Etiology3
Genetic Factors
- Mutations in cardiac sarcomere protein genes
- Multiple modes of inheritance, but most commonly autosomal dominant
Environmental Factors
- Viral myocarditis (e.g., influenza, herpes, and adenovirus) can lead to DCM.
- Toxins (e.g., anthracycline exposure during chemotherapy)
Other Causes
- Inborn errors of metabolism
- Neuromuscular disorders
Pathophysiology and Diagnosis1-4
Dilated Cardiomyopathy
- Dilated CM is characterized by depressed ventricular function secondary to ineffective systolic shortening.
- Transthoracic echo (TTE): Dilatation of four heart chambers, primarily the left ventricle (LV); reduced ventricular systolic function; mitral and/or tricuspid valve regurgitation from annular dilation.
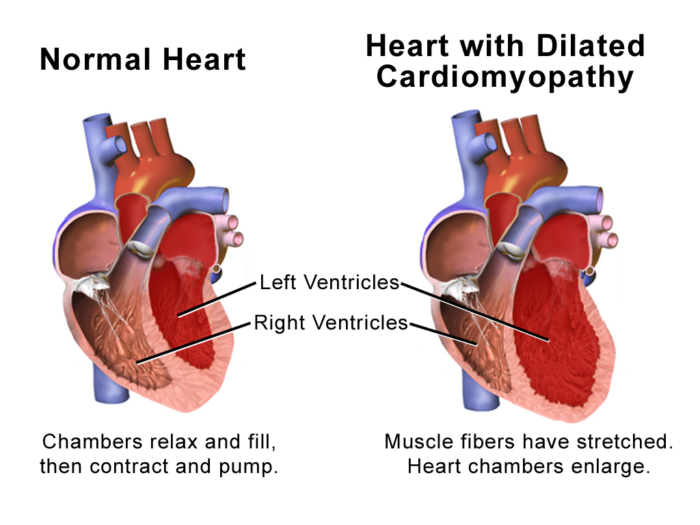
Figure 1. Heart with DCM. Image courtesy of Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014”. WikiJournal of Medicine 1 (2). Link.
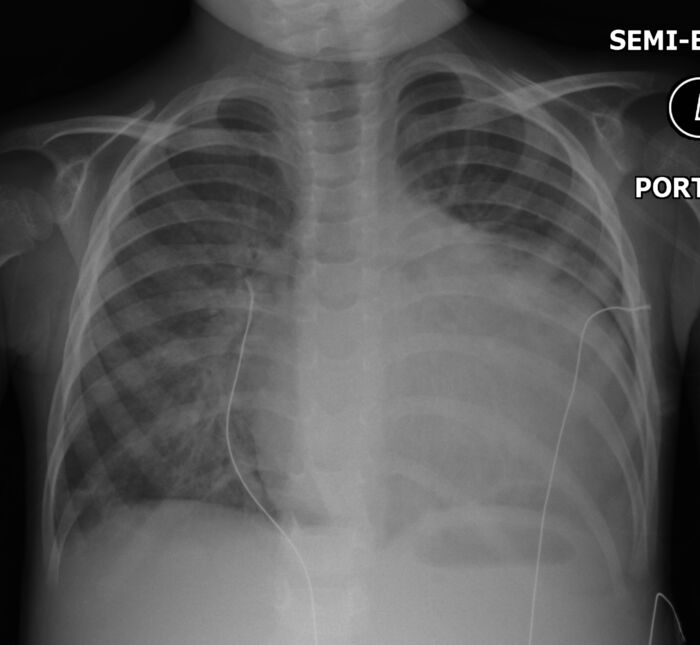
Figure 2. CXR demonstrating marked cardiomegaly in the setting of DCM. Case courtesy of Hani Makky Al Salam, Radiopaedia.org, rID: 13519. Link.
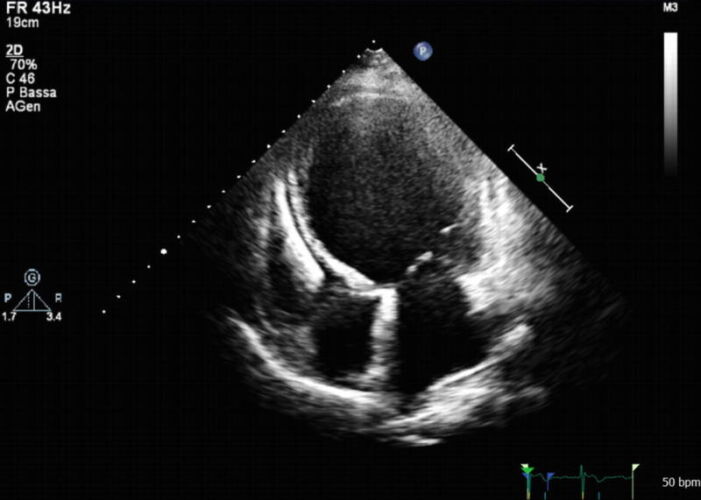
Figure 3. TTE demonstrating severe LV dilatation in DCM. Source: Pinamonti B, et al. Role of cardiac imaging: Echocardiography. 2019. In: Sinagra G, Merlo M, Pinamonti B, editors. Dilated Cardiomyopathy: From Genetics to Clinical Management [Internet]. Cham (CH): Springer; 2019. Fig. 7.2. CC BY 4.0. Link.
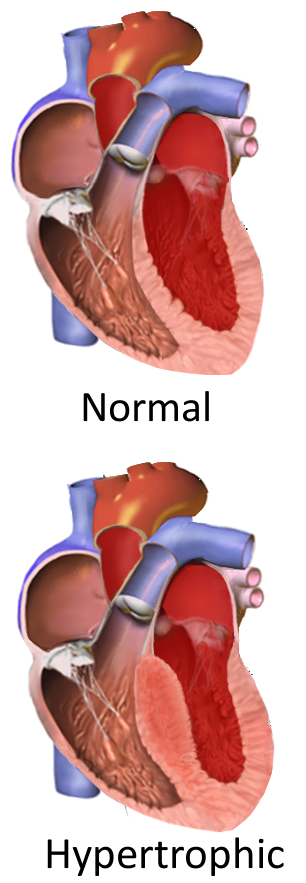
Hypertrophic Cardiomyopathy
- Characterized by asymmetric septal hypertrophy and systolic anterior motion (SAM) of the mitral valve, resulting in dynamic left ventricular outflow tract obstruction (LVOTO).
- TTE: Hypertrophied, nondilated ventricle with preserved ventricular systolic function and impaired diastolic myocardial relaxation. In pediatrics, the criterion for diagnosis is a maximal LV wall thickness with a Z score > 2, corrected for body surface area.
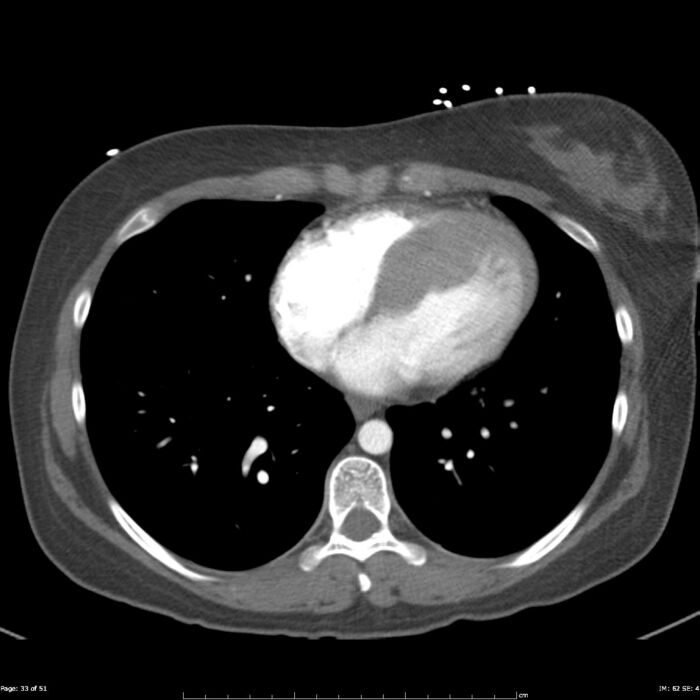
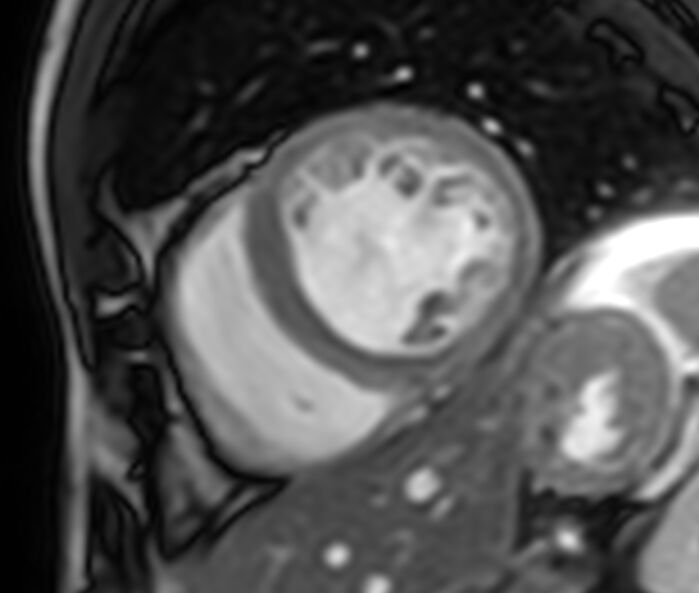
Figure 5. MRI demonstrating HCM, in this case asymmetrically involving the septum. Case courtesy of Frank Gaillard, Radiopaedia.org, rID: 23727. Link.
Restrictive Cardiomyopathy
- Restrictive CM is characterized by noncompliant myocardium without ventricular hypertrophy.
- TTE: Near-normal ventricles with poor diastolic ventricular relaxation and marked bilateral atrial enlargement; presence of intraventricular thrombi
Noncompaction Cardiomyopathy
- Noncompaction CM is characterized by abnormal ventricular development results in excessive and deep trabeculation.
- TTE: Prominent LV trabeculation, decline in systolic and/or diastolic function
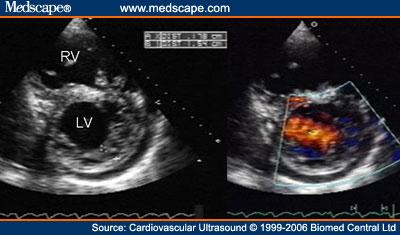
Figure 7. Echo demonstrating NCM. Espinola-Zavaleta N, Soto ME, Castellanos LM, Játiva-Chávez S, Keirns C., CC BY 2.0, via Wikimedia Commons. Link.
Arrhythmogenic Right Ventricular Dysplasia
- This condition is characterized by fibro-fatty infiltration of the myocardium that creates arrhythmogenic foci, mostly involving the RV free wall.
- TTE: Regional wall hypokinesis
Clinical Presentation of Pediatric Cardiomyopathies
- Variable clinical presentation, ranging from asymptomatic to overt heart failure
- Adolescents: Exercise intolerance, dyspnea, chest pain, syncope, and dysrhythmia
- Children: Asthma-like symptoms or gastrointestinal distress (abdominal pain, nausea/vomiting) and inability to keep up with their peers
- Infants: Failure to thrive, tachypnea, and diaphoresis with feeding
- Asymptomatic does not imply mild disease; patients can present with sudden cardiac arrest without a previous diagnosis.
Prognosis2,3
- Outcome varies, with five-year survival (free of death or transplantation) of 60%, 95%, and 68% for DCM, HCM, and RCM, respectively.
Treatment Strategies
Medical Management
- Medications:
- Control/treat heart failure: Diuretics, beta-blockers, digoxin, calcium channel blockers
- Reverse ventricular remodeling: Angiotensin-converting enzyme inhibitor (ACEi)
- Antiarrhythmic drugs
- Anticoagulants
- Lifestyle modification: Dehydration, caffeine, and competitive sports should be avoided.
Surgical/Device Management
- Automatic implantable cardioverter-defibrillator (AICD): AICD prevents sudden cardiac death (SCD) in patients at high risk for malignant arrhythmias.
- Mechanical support with ventricular assist device (VAD): bridge to transplant or destination therapy
- Surgery
- Surgical myectomy for HCM
- Heart transplantation
Anesthetic Considerations
Preoperative Assessment1,3,4
- A complete history and physical examination should be performed focusing on signs and symptoms of heart failure and comorbidities such as neuromuscular disorder and genetic/metabolic disease.
- Baseline functional status should be assessed:
- Older patients: presence and severity of dyspnea, exercise intolerance, chest pain, syncopal episode, palpitations, and weight gain
- Infants/children: failure to thrive, tachypnea, gastrointestinal distress, diaphoresis with feeding, fatigue, low activity levels
- Current medications should be reviewed.
- Electrolyte levels should be checked, and hypokalemia corrected in patients who are on diuretics.
- Beta-blockers, calcium channel blockers, and antiarrhythmic medications should be continued.
- ACEi should be withheld on the morning of the procedure to avoid intraoperative hypotension.
- The AICD should be interrogated to ensure proper functioning. An electrophysiologist should be consulted to reprogram the AICD, if needed.
- Chest radiographs, baseline electrocardiogram, and the most recent echocardiogram should be reviewed. In addition, catheterization data, any other imaging data, and laboratory investigations should be evaluated.
- The eligibility of extracorporeal support should be discussed with the patient’s family and primary cardiologist in patients with end-stage CM requiring general anesthesia.
- A cardiologist may be consulted to optimize the patient’s medical condition as needed.
- The patient’s heart failure team/cardiologist should be aware that the patient is undergoing a procedure.
Intraoperative Management1,3,4
- Reliable intravenous (IV) access should be established before induction of general anesthesia.
- In addition to standard American Society of Anesthesiologists monitors, 5-lead electrocardiogram and arterial line monitoring should be considered in potentially unstable patients.
- External defibrillation pads may be placed before induction in patients with a history of dysrhythmia or have their AICD deactivated.
- Medication should be slowly titrated, allowing sufficient time to take effect due to prolonged circulation time in patients with low cardiac output (CO).
- There is no single best anesthetic technique for patients with CM. An induction plan should be made based on the severity of the cardiac condition. Depth of anesthesia should be maintained to facilitate the necessary procedure and sedation techniques should be used when appropriate. The overlying goal is maintaining CO while minimizing any increase in oxygen consumption.
- Hemodynamic goals vary for the different types of CM:
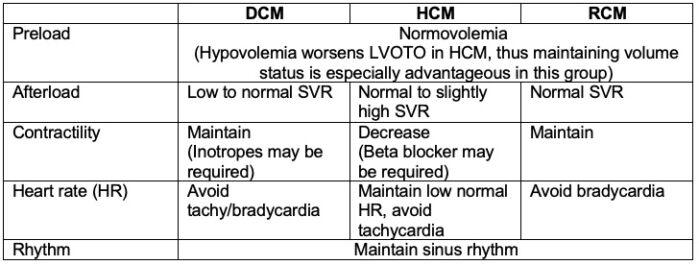
Table 1. Hemodynamic goals for the different types of CM
Specific Considerations1-4
DCM
- An increase in SVR should be avoided since a poorly contractile left ventricle will not maintain CO. Inotropes with vasodilation properties such as milrinone, dobutamine, and low-dose epinephrine should be considered to maintain the CO.
- High doses of inotrope may be detrimental to end-stage DCM because resulting tachycardia can increase myocardial oxygen consumption. Furthermore, the heart may be unable to increase contractility in response to increasing inotrope dose.
- An enlarged heart may cause extrinsic left main bronchus compression requiring positive end-expiratory pressure to overcome the obstruction.4
HCM
- Patients with HCM are predisposed to perioperative arrhythmias and myocardial ischemia in the presence of tachycardia and low diastolic arterial pressures.
- Prevention of hypovolemia, maintaining slightly elevated SVR, and avoiding a state of increased myocardial contractility with low normal heart rate are the keys to minimizing LVOTO.
- Inotropes (e.g., epinephrine, dobutamine) should be used with caution, as they may decrease the CO due to ventricular cavity obliteration from hypercontractility and decreased diastolic filling time, leading to worsening LVOTO.
- Hypotension should be treated by ensuring adequate preload and administering vasopressors (e.g., phenylephrine, vasopressin).
- Tachycardia should be promptly treated with a short-acting beta-blocker, such as esmolol.
- Propofol as a sole anesthetic agent should be avoided as a decreased SVR is not well tolerated.
RCM
- Bradycardia should be avoided as the CO depends on HR and preload.
- Iatrogenic increase in pulmonary vascular resistance (PVR) from hypoxia, hypercarbia, acidosis, high airway pressure, hypothermia should be avoided. Noncompliant ventricles lead to progressively elevated left ventricular end-diastolic pressure (LVEDP) and left atrial pressure (LAP), resulting in pulmonary venous congestion leading to an elevated PVR. Thus, patients usually present with pulmonary hypertension at the time of diagnosis.
AVRD
- Drugs with sympathomimetic effects and exogenous catecholamines should be avoided.
- The dose of bupivacaine should be reduced and epinephrine in local anesthetics should be avoided when performing regional anesthesia.
Postoperative Management
- Adequate pain control using multimodal analgesia should be used to avoid tachycardia.
- Stable patients undergoing minimally invasive procedures can be recovered in the postanesthesia care unit.
- For invasive surgeries and/or unstable patients, the recovery should take place in the intensive care unit.
- AICD should be reprogrammed immediately after the operation.
- Close observation for the signs of myocardial ischemia and arrhythmias is critical.
References
- Pearce B. Hypertrophic cardiomyopathy. In: Berenstain L.K. and Spaeth J.P. Congenital Cardiac Anesthesia. New York; Cambridge University Press; 2021: 113-20.
- Ing RJ, Ames WA, Chambers NA. Paediatric cardiomyopathy and anaesthesia. Br J Anaesth. 2012;108(1):4-12. PubMed
- Lee TM, Hsu DT, Kantor P, et al. Pediatric Cardiomyopathies. Circ Res. 2017;121(7):855-73. PubMed
- Alcos S, Loepke AW. Dilated cardiomyopathy. In: Berenstain LK and Spaeth JP. Congenital Cardiac Anesthesia. New York; Cambridge University Press; 2021: 246-51.
Other References
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.