Copy link
Pediatric Anemia
Last updated: 10/03/2025
Key Points
- Anemia in pediatric patients is common in the perioperative period and can lead to increased morbidity and mortality. Understanding the classification and underlying etiology can provide insight for perioperative management.
- While physiologic anemia is common in infants, the most common cause of anemia in most pediatric populations is iron deficiency.
- A transfusion of red blood cells should be considered when hemoglobin falls below 7 g/dL or below 9-11 g/dL in neonates.
- Red blood cell transfusions of 10 mL/kg should increase hemoglobin by 2 g/dL.
Introduction
- Anemia is the most common hematologic disorder in children and can lead to increased morbidity and mortality in the perioperative setting.1
- Anemia is defined as a reduction in the hemoglobin level or red blood cell mass resulting in the inability to meet necessary oxygen demands. This generally correlates to hemoglobin values less than two standard deviations below the mean or less than the 2.5th percentile based on a patient’s age and sex.2 See Table 1 for values.
- Term neonates have higher hemoglobin levels than older children and adults (Figure 1). A physiologic anemia occurs with term infants reaching a hemoglobin nadir at 8-12 postnatal weeks. Hemoglobin then steadily increases during childhood until it reaches adult levels.3
- The most common causes of pediatric anemia vary by age, with iron deficiency contributing to a large number of cases.
- Treatment of anemia varies based on cause and acuity, and it can include dietary changes, red blood cell transfusions, or even surgical intervention.
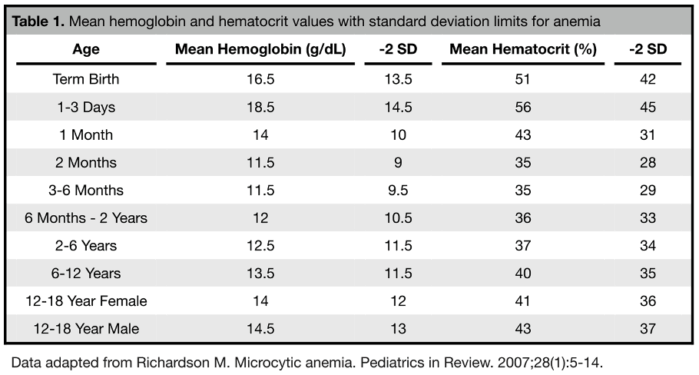
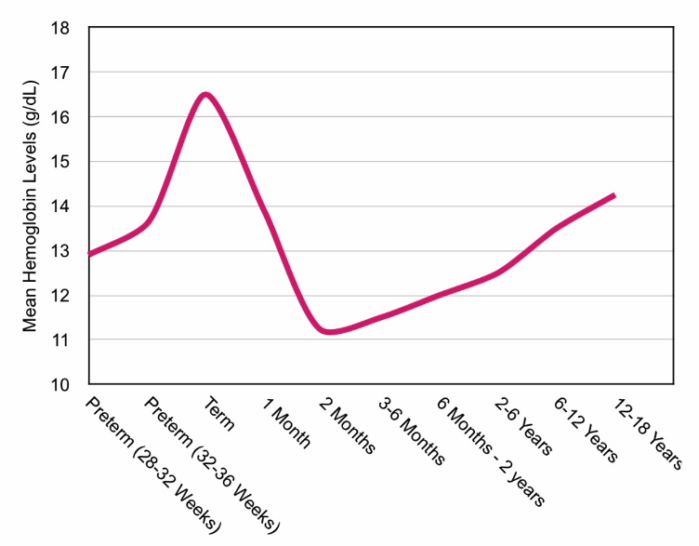
Figure 1. Mean hemoglobin levels by age. Data adapted from Wang M. Iron deficiency and other types of anemia in infants and children. Am Fam Physician. 2016;93(4):270-278 and Adams TL, et al. Essentials of Hematology. In: Cote CJ, Lehman J, et al., eds. A Practice of Anesthesia for Infants and Children. 6th ed. Philadelphia; Elsevier; 2019:217-239.
Causes of Pediatric Anemia
Physiologic Anemia of the Neonate
- Physiologic anemia is the most common cause of anemia in neonates and young infants.
- As tissue oxygenation abruptly increases after birth, erythropoiesis decreases. In addition, neonatal red blood cells have a shorter lifespan.
- Term infants reach a generally asymptomatic physiologic nadir of 10-12 g/dL by 8-10 weeks of life. Preterm infants may see a more drastic decline to 7-8 g/dL, which may become symptomatic. The level of severity correlates with the prematurity of the patients.4
Iron Deficiency Anemia (IDA)
- IDA is the most common type of anemia in children, with a prevalence of 1-2% in young children.5
- Risk factors for IDA include prematurity, low socioeconomic background, poor diet, chronic blood loss, and malabsorption disorders.6
- Testing for IDA includes iron studies and ferritin levels. IDA is usually a microcytic anemia, but can be normocytic in some patients.5
- Prevention of IDA in neonates and infants includes iron supplementation in those exclusively breastfed and delayed umbilical cord clamping.5
- Treatment is usually with a change in diet or oral supplementation. Severe or refractory IDA, intravenous iron supplementation may be necessary.6
Other Nutritional Anemias
- Vitamin B12 deficiency is a rare macrocytic anemia that can occur due to decreased intake, poor absorption, or problems with transport or metabolism. Symptoms can include developmental delays and neurologic issues. Deficiencies are treated with supplementation.6
- Folate deficiency due to decreased intake or absorption, increased requirement, or decreased utilization (such as in the use of folic acid antagonists) can result in megaloblastic anemia. Children with chronic hemolytic disorders or those undergoing hemodialysis are at increased risk. Folate deficiency is treated with supplementation, changes in diet, or cessation of the offending drug.2
Sickle Cell Disease
- Sickle cell disease is the most common group of inherited blood disorders, with sickle cell anemia (HbSS) being the most common.6 Milder forms of sickle cell disease include sickle cell/hemoglobin C (HbSC) and sickle cell/beta thalassemia (HbSβ).3
- Hemoglobin S (HbS) is caused by a mutation in the β-globin gene on chromosome 11, leading to a glutamate to valine substitution. Homozygous inheritance of HbS leads to sickle cell anemia, resulting in polymerization of hemoglobin in deoxygenated conditions, as well as in the setting of acidosis, dehydration, hypothermia, and inflammation.3
- Once erythrocytes become sickled, vaso-occlusion, hemolysis, coagulation, and inflammatory pathway activation, endothelial dysfunction, and tissue damage can occur.3
- Sickle cell anemia affects most organ systems and manifests as hemolytic anemia, acute pain crises, acute chest syndrome, infection, osteonecrosis, renal insufficiency, cholelithiasis, pulmonary hypertension, splenic dysfunction, skin ulcerations, or cerebrovascular events. Disease severity is variable, and the clinical course is progressive.3
- Treatment with hydroxyurea and folate supplementation is recommended. Symptoms are controlled with a multimodal pain regimen, chronic transfusions, hydration, oxygen support, and antibiotics as necessary. Hematopoietic stem cell transplants are a possible cure for sickle cell anemia.3
- Perioperative management includes adequate hydration, oxygen supplementation, transfusion with a goal hemoglobin level of ~10g/dL prior to moderate and high-risk procedures, and close postoperative monitoring.3
- Please see the OA summary on sickle cell disease for further details. Link
Other Hemoglobinopathies
- Alpha thalassemia is caused by alpha-globin gene deletions, resulting in excess beta globin chains. Disease severity depends on the number of alleles affected. Deletion of 4 genes is the most severe form, resulting in hydrops fetalis. A single alpha gene deletion is generally asymptomatic, and symptomatic forms can be associated with hemolysis and severe anemia.6
- Beta thalassemia is caused by beta globin gene mutations with variable presentation. Symptomatic forms may require lifelong blood transfusions and can be associated with poor growth, skeletal abnormalities, chronic iron overload, splenomegaly, and cardiac failure.6
- Please see the OA summary on thalassemia for further details. Link
Membranopathies
- Hereditary spherocytosis is a form of chronic hemolysis due to abnormalities in erythrocyte membrane proteins, causing a spherical shape. Signs and symptoms vary by disease severity and include anemia, jaundice, splenomegaly, growth failure, skeletal abnormalities, disseminated intravascular coagulation, and pulmonary hypertension. Some patients may become transfusion dependent. Splenectomy can increase the life span of the erythrocytes and may be considered for pediatric patients with severe disease.3,6
- Hereditary elliptocytosis disorders are due to membrane protein defects causing an elliptical shape of erythrocytes. Most types are asymptomatic outside of infancy, but others can result in severe hemolytic anemia.6
Enzymatic Disorders
- Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common enzyme deficiency that results in hemolysis in the setting of oxidative stress. The neonatal form causes jaundice which may require phototherapy or exchange transfusion in severe cases. Other forms cause chronic anemia or episodic hemolysis precipitated by stress.6 In the perioperative period, avoidance of triggers such as prilocaine, benzocaine, methylene blue, procainamide, hydralazine, and sodium nitroprusside is necessary.3
- Pyruvate kinase deficiency is an abnormality in the glycolysis pathway leading to hemolytic anemia. Typically presenting in infancy, severity ranges from compensated anemia to transfusion dependence. Splenectomy may be indicated.6
Other Hemolytic Anemias
- Transfusion-associated hemolytic anemia can be acute or delayed, caused by either ABO incompatibility or antibodies against non-ABO erythrocyte antigens. Treatment is generally supportive. Please see the OA summary on blood transfusion reactions for further details. Link
- Primary autoimmune hemolytic anemias (AIHA) generally occur in the absence of systemic illness. Most forms are IgG or IgM mediated, but can also be IgA mediated. Hemolysis can be intravascular or extravascular depending on the type, but primary AIHA is generally self-limited. Treatment for severe cases includes steroids, IVIG, various immunomodulators, or even splenectomy.6
- Secondary AIHA occurs in the setting of systemic illness or drug exposure. Some causes of secondary AIHA include viral infections, autoimmune disease, malignancy, and immunodeficiency syndromes. Drugs that may cause AIHA include ceftriaxone, piperacillin, and diclofenac. Treatment is similar to that of primary AIHA, in addition to treatment of the causative illness.6
- Neonatal alloimmune hemolytic anemia occurs due to maternal antibodies that attack incompatible fetal or neonatal erythrocyte antigens. Rh sensitization leading to hemolysis has largely been prevented with the use of anti-D immunoglobulin prophylaxis (RhoGam), but other incompatibilities still remain an issue.6
- Disseminated intravascular coagulopathy (DIC) is a microangiopathic hemolytic anemia associated with coagulation activation, fibrinolysis, and consumption of platelets. In the pediatric population, some causes of DIC include sepsis, malignancy, liver failure, and necrotizing enterocolitis. Treatment is supportive.6
- Hemolytic uremic syndrome (HUS) is a microangiopathic hemolytic anemia associated with thrombocytopenia and renal injury. Often caused by E. coli shiga-like toxins, HUS may present with abdominal pain, emesis, jaundice, hematuria, or bloody diarrhea and may progress to renal failure.6
Bone Marrow Failure
- Infectious suppression of the bone marrow leading to anemia can occur after several viral infections, including parvovirus B19, Epstein-Barr virus, and human immunodeficiency virus, as well as several bacterial infections. Patients with other forms of chronic anemia can be at risk of severe decompensation in the event of infectious bone marrow suppression.2
- Transient erythroblastopenia of childhood is a selective erythrocyte aplasia that can occur after a viral infection. It is a self-limited anemia thought to be immune-mediated.2
- Bone marrow failure and pancytopenia can develop due to infiltration of the medullary space by other disease processes. These include leukemia, neuroblastoma, certain storage diseases, granulomatous lesions, and myelofibrosis. Treatment is aimed at treating the underlying condition.6
- Fanconi anemia is caused by gene defects leading to pancytopenia. Most patients will have other congenital defects and may eventually progress to bone marrow failure. In addition to anemia, patients will have frequent infections and bleeding. Stem cell transplants may be curative.6
- Diamond-Blackfan anemia is a macrocytic, congenital red cell aplasia that results from ribosomal protein gene mutations. Mostly diagnosed in infancy, this anemia is associated with other congenital anomalies and an increased risk for malignancy. Treatment is with steroids, transfusions, and a stem cell transplant.2
Other Causes of Anemia
- Lead poisoning can interrupt heme biosynthesis, leading to anemia. Children are primarily exposed to lead through the ingestion of lead-contaminated paint chips and dust, as well as through ceramic pots and imported foods. Symptoms are non-specific, so there are recommendations for screening children with certain risk factors.2
- Anemia of chronic disease can present in patients with a multitude of conditions, including heart disease, lung disease, inflammatory bowel disease, rheumatologic disorders, thyroid disorders, and chronic kidney disease. This anemia is typically normocytic and erythropoietin levels are low. The exact cause differs, but includes hemolysis, decreased erythropoietin sensitivity, and increased hepcidin, interfering with iron transport.6
- Chronic blood loss from sources such as the gastrointestinal tract, chronic epistaxis, or dysmenorrhea can also lead to anemia. This should be on the differential if patients have risk factors.
- Many medications and toxins can cause either hemolytic anemia or transient bone marrow suppression. Common drugs include analgesics (indomethacin, diclofenac, acetaminophen), antimicrobials (penicillin, cephalosporins, sulfas), antiepileptics (valproic acid, phenytoin, carbamazepine), and antineoplastics. Toxins include insecticides, pesticides, and arsenic. Anemia usually resolves with the removal of the offending agent.2
Presentation and Workup
- Anemia can present in different ways depending on the severity and duration. Chronic anemia can lead to pallor, fatigue, dyspnea, dizziness, syncope, failure to thrive, and changes in neurocognitive development. If anemia is due to hemolysis, jaundice or dark urine may be present.6
- Acute, severe anemia can present with hypoxia, hypotension, tachycardia, tachypnea, seizures, congestive heart failure, and other life-threatening symptoms.6
- While the American Academy of Pediatrics recommends testing for anemia at 12 months of age, the United States Preventive Services Task Force concludes that there is insufficient evidence for screening in children 6-24 months of age. Despite this, it is generally recommended to screen for anemia in high-risk patients, those who are symptomatic, or those who will undergo surgeries with a high risk for blood loss.7
- After an initial complete blood count determines anemia, further testing, such as a peripheral blood smear, reticulocyte count, direct antiglobulin test, bilirubin levels, iron studies, hemoglobin electrophoresis, or lead levels, may be useful in determining the cause of anemia.6
Management of Pediatric Anemia in the Perioperative Setting
Preoperative Management
- Management of anemia should start with preoperative optimization.
- Patients undergoing surgical procedures with a high risk of blood loss should be screened for anemia, ideally 3-6 weeks before elective surgeries. It is reasonable to delay elective surgery for optimization.
- As iron deficiency is a major cause of anemia in pediatric patients, oral iron supplementation is the first line therapy. Oral iron supplementation is expected to increase hemoglobin levels by 1 g/dL over 4-6 weeks. Intravenous iron supplementation is an option for patients who do not tolerate oral therapy, for those with more severe anemia, or for those who require more urgent surgeries.1
Intraoperative Management
- Due to the differences in the intravascular volume of pediatric patients (Table 2), great care should be taken to avoid fluid overload leading to possible hemodilutional anemia and coagulopathy.
- Strategies to reduce intraoperative blood loss should be considered during high-risk surgeries, including scoliosis, maxillofacial, craniofacial, cardiac, and hepatic surgeries.
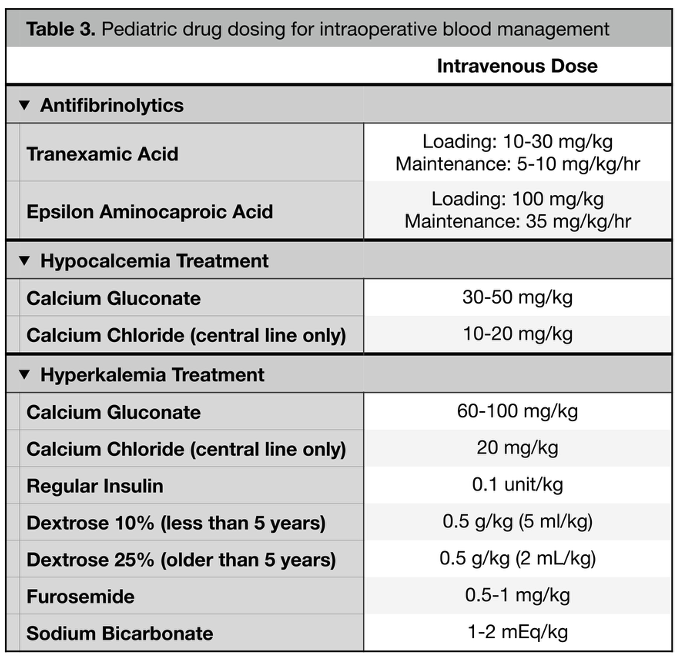
- Antifibrinolytic therapy with tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) is one method to reduce blood loss (Table 3). TXA should be administered as a loading dose of 10-30 mg/kg followed by an infusion of 5-10 mg/kg/hr. EACA is administered as a loading dose of 100 mg/kg followed by an infusion of 35 mg/kg/hr.1
- Cell salvage is another method for reducing red blood cell transfusion in pediatric patients and is generally used for patients >10 kg.1
- Goal-directed transfusion therapy based on laboratory data and hemodynamic parameters should be implemented whenever possible. It is also reasonable to implement the use of near-infrared spectroscopy as a measure of oxygen delivery and transfusion necessity, especially in the neonatal population.
- Similar to adults, the recommended transfusion threshold for most hemodynamically stable pediatric patients is 7 g/dL with a goal of 9.5 g/dL once transfusion has been initiated (Figure 2).8
- Neonatal patients generally require a hemoglobin goal of 9 g/dL. Term neonates in the first week of life, intubated neonates, or those with an oxygen requirement should be maintained at a hemoglobin of 11 g/dL.9 Other pediatric populations, such as those with cardiac disease, traumatic brain injury, sickle cell disease, or certain malignancies, may also require higher hemoglobin levels.8
- Transfusions of packed red blood cells should start at 10 mL/kg and are expected to increase hemoglobin by ~2 g/dL.
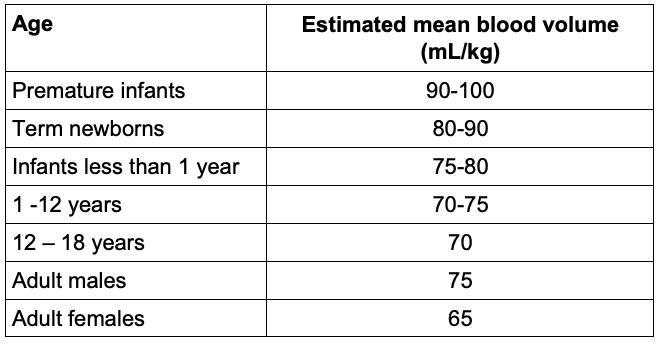
Table 2. Estimated blood volumes for different age groups.10
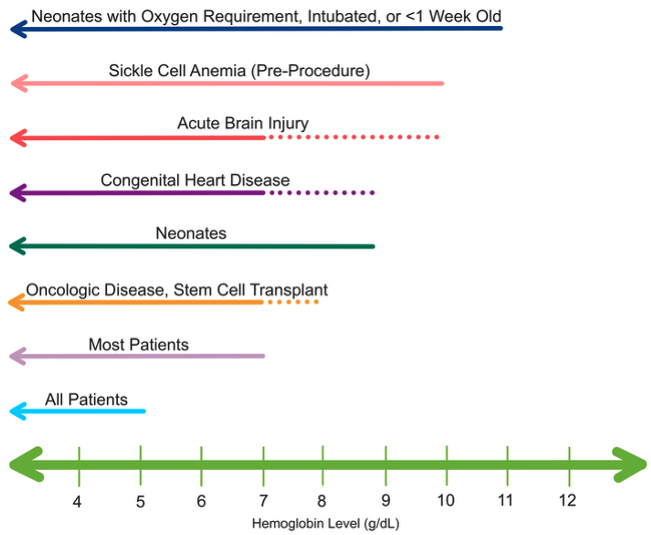
Figure 2. Transfusion thresholds in hemodynamically stable pediatric patients who are currently with or at risk for critical illness.8,9
Postoperative Management
- Anemia is likely a risk factor for apnea of prematurity as well as postoperative apnea in infants. Guidelines recommend at least 12 hours of postoperative monitoring for neonates and former preterm infants based on current age and risk factors. Please see the OA summary on apnea of prematurity and postoperative apnea for more details. Link
- Blood draws for laboratory studies are a cause of anemia in pediatric patients, especially in the postoperative period. While the use of smaller blood tubes may help mitigate iatrogenic anemia, lab studies should be limited as much as possible to avoid further transfusion needs.
Pediatric Transfusion Concerns
- Pediatric patients, especially neonates, are at an increased risk of life-threatening hyperkalemia due to blood transfusions. Methods to mitigate the risk include avoiding rapid transfusion, administration through peripheral rather than central access, and reducing the storage time of products.11 See Table 3 for pediatric dosing of hyperkalemia treatment.
- The immature cardiovascular system of neonates increases the risk of transfusion-related adverse events. Transfusion-induced hypocalcemia should be avoided as neonates are more reliant on extracellular calcium for cardiac function compared to older patients. Neonates also have a reduced ability to augment cardiac output in the setting of increased preload. The rapid transfusion of blood products can more easily lead to fluid overload and cardiac dysfunction.12 Please see the OA summary on neonatal cardiovascular physiology for more details. Link
- Pediatric patients are more prone to intraoperative hypothermia, so red blood cell transfusion should be administered with a fluid warmer to prevent further derangements.
- While some blood protocols call for irradiation of products for neonatal transfusion to reduce the risk of transfusion-associated graft-versus-host disease, the Association for the Advancement of Blood and Biotherapies recommends irradiation only for fetal or neonatal recipients of intrauterine transfusions or for those receiving a transfusion from a blood relative.13 Extremely premature neonates may also benefit from receiving irradiated products, as well as pediatric patients with hematologic malignancies or stem cell transplants. Be aware that irradiation may elevate the potassium level of the blood product. Please see the OA summary on blood product modifications for more details. Link
References
- Goobie SM, Faraoni D. Perioperative paediatric patient blood management: a narrative review. Br J Anaesth. 2025;134(1):168-179. PubMed
- Martinez-Torres V, Torres N, Davis JA, Corrales-Medina FF. Anemia and associated risk factors in pediatric patients. Pediatric Health Med Ther. 2023; 14:267-280. PubMed
- Adams TL, Latham GJ, Eisses MJ, Bender MA, Haberkern CM. Essentials of Hematology. In: Cote CJ, Lehman J, Anderson BJ, eds. A Practice of Anesthesia for Infants and Children. 6th ed. Elsevier; 2019:217-239. Link
- Widness JA. Pathophysiology of anemia during the neonatal period, including anemia of prematurity. Neoreviews. 2008;9(11):e520. PubMed
- Wang M. Iron deficiency and other types of anemia in infants and children. Am Fam Physician. 2016;93(4):270-8. PubMed
- Gallagher PG. Anemia in the pediatric patient. Blood. 2022;140(6):571-593. PubMed
- Siu AL. Screening for iron deficiency anemia in young children: USPSTF Recommendation Statement. Pediatrics. 2015;136(4):746-52. PubMed
- Valentine SL, Bembea MM, Muszynski JA, et al. Consensus recommendations for RBC transfusion practice in critically ill children from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatric Critical Care Medicine. 2018;19(9):884-98. PubMed
- Pilkington M, Nelson G, Pentz B, et al. Enhanced Recovery After Surgery (ERAS) Society recommendations for neonatal perioperative care. JAMA Surg. 2024;159(9):1071-8. PubMed
- Riley AA, Arakawa Y, Worley S, et al. Circulating blood volumes: A review of measurement techniques and a meta-analysis in children. ASAIO Journal. 2010;56(3): 260-4. PubMed
- Burke M, Sinha P, Luban NLC, Posnack NG. Transfusion-associated hyperkalemic cardiac arrest in neonatal, infant, and pediatric patients. Front Pediatr. 2021; 9:765306. PubMed
- Cooper DS, Hill KD, Krishnamurthy G, et al. Acute cardiac care for neonatal heart disease. Pediatrics. 2022;150(Supplement 2). PubMed
- Association for the Advancement of Blood & Biotherapies, ARC, America’s Blood Centers, Armed Services Blood Program. Circular of information for the use of human blood and blood components. Irradiation. 2021. p40. Link
Other References
- Manohar C, Knuf K. Perioperative anemia. OA summary. Published January 27, 2023. Accessed October 3, 2025. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.