Copy link
Oliguria in the Perioperative Setting
Last updated: 05/24/2023
Key Points
- Oliguria in adults is typically defined as a urinary output less than 0.5 ml/kg/hour lasting for at least 6 hours.
- Individualized plans for fluid monitoring should be utilized to avoid excess administration of fluids in response to oliguria.3
Introduction
- Oliguria is typically defined as a urinary output less than 0.5 mL/kg/hour lasting for at least 6 hours. It is a common finding during routine preoperative evaluation.1
- Urine output may be influenced by baseline hemodynamics, intravascular volume status, hormone levels, and medications, including anesthetic agents that affect renal blood flow and glomerular filtration rate.
- Durations of low urine output longer than 120 minutes are associated with a higher risk for developing kidney injury, thereby increasing the likelihood of postoperative complications, length of hospital stay, and mortality.2
- Oliguria, with an estimated incidence of 12% of hospital admissions, is an early indicator of acute renal injury, which results in a significantly higher risk for morbidity, as well as mortality.3
- Approximately half of the patients admitted to the intensive care unit (ICU) experience episodes of oliguria.3
- Acute oliguria can lead to a variety of complications including electrolyte imbalances, neurologic dysfunction, cardiovascular instability, gastrointestinal distress, respiratory insufficiency, and anemia.
Etiology
- Oliguria may be due to an appropriate physiological response (i.e., retaining water in the case of dehydration) or it may be caused by an underlying pathology that affects the kidneys or urinary tract.1
- Physiological mechanisms for conserving fluid and electrolytes are tightly regulated by neurohormonal control and can be reversed without subsequently injuring the kidneys.
- Oliguria may be caused by a variety of etiologies that can be classified into three general categories: prerenal, renal, and postrenal.1
- Low urine output is frequently observed during the standard postoperative course as a consequence of increased vasopressin release and sympathetic stimulation during surgery.
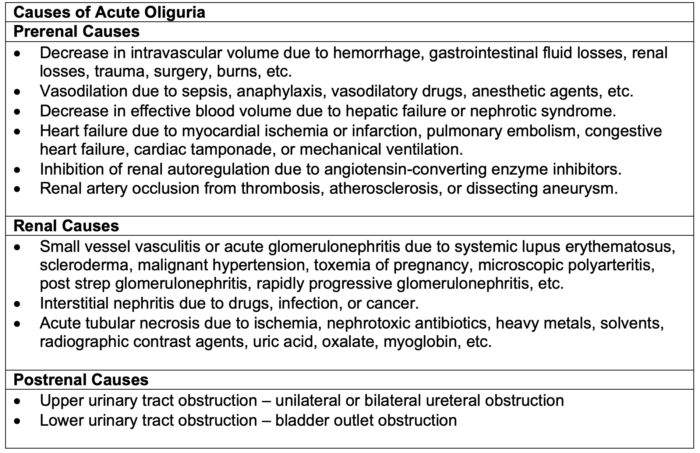
Table 1. Causes of acute oliguria. Adapted from Klahr S, Miller SB. Acute oliguria. N Engl J Med. 1998.1
Evaluation
When oliguria is observed or suspected, evaluation should begin with a comprehensive history (medications, occupation, recent travel) and physical exam (skin turgor, mucous membranes), followed by basic laboratory workup.
- If it is evident that a patient has not been able to adequately hydrate independently, then hypovolemia is the presumed diagnosis unless the condition remains unchanged after adequate fluid replacement.
- Baseline workup should include urinalysis, serum creatinine, urea, serum electrolytes, and blood urea nitrogen, as well as renal ultrasound if an obstructive pathology is suspected.
- Specific situations may also require an analysis of autoimmune etiologies (i.e., complement levels, ANA, and ANCA).3
- Postrenal causes are common in the hospital setting and are often caused by obstructed catheters that can be corrected using irrigation or readjustment of equipment.
- Prompt analysis is necessary in the setting of oliguria to prevent the possibility of progression to acute renal failure.4
- If the hemodynamic status cannot be adequately monitored noninvasively, then an arterial line or central venous catheter (CVC) may be required.
- Specific gravity, urine sodium concentration, and fractional excretion of sodium may all be used to further evaluate the underlying cause of oliguria.
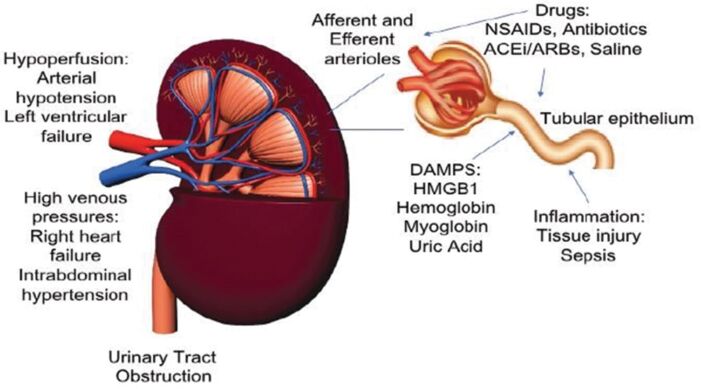
Figure 1. Major mechanisms of acute kidney injury in the perioperative setting. Used with permission from Zarbock A, et al. Update on perioperative acute kidney injury. Anesth Analg. 2018.6
Fractional Extraction of Sodium (FENa)
- Calculating FENa can be helpful in identifying the cause of oliguria. FENa measures the percent of filtered sodium that is excreted in urine. It is a measurement of the ability of the kidney to extract sodium and water from the glomerular filtrate. It is a ratio of the sodium filtration to the overall GFR, which is estimated by the renal creatinine filtration. In other words, how much sodium is excreted into the urine relative to the total amount of sodium passing through the kidney. The calculation for FENa is as follows:
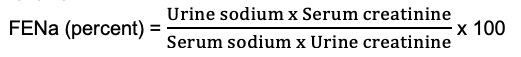
- FENa less than 1% suggests a prerenal cause of oliguria. The intact proximal tubules are able to reabsorb sodium resulting in a very low urine sodium concentration. There is also a stimulus to conserve or reabsorb sodium, and water with it, suggesting that the kidneys sense poor perfusion or decreased arterial volume. Poor perfusion may be the result of hypovolemia, renal vasoconstriction or renal artery stenosis, reduced cardiac function, or decreased GFR. If the patient is truly hypovolemic, kidney function may improve with fluid resuscitation. Low FENa can also be seen in other states with low urine sodium in patients who are not volume-depleted such as acute glomerulonephritis, sepsis, cardiorenal syndrome, hepatorenal syndrome, contrast-induced nephropathy, etc.
- Healthy euvolemic patients with normal renal function and dietary sodium intake will have a FENa of approximately 1%.
- FENa greater than 2% suggests an intrinsic insult to the kidneys, such as acute tubular necrosis or interstitial nephritis, where the proximal tubules do not reabsorb sodium from the glomerular filtrate, resulting in high urinary sodium.
- A FENa value between 1 and 2% may be seen with either disorder.
- FENa is less accurate and should not be used in patients with known chronic kidney disease, obstruction, acute glomerular disease, or patients on diuretics. Fractional extraction of urea is preferred in patients on diuretics.
Management
The management of patients experiencing oliguria will depend on the underlying etiology elucidated in the steps above. However, the following treatment strategies may be utilized in a stepwise approach for all cases.
1. The patient’s hemodynamics should be stabilized using balanced crystalloids.
-
- Intravenous fluids should be titrated based on mean arterial pressures with a goal of 65-70 mmHg.3
- Urine output should be monitored hourly to avoid volume overload.4
2. If crystalloids do not improve urine output but the patient appears euvolemic, diuretic therapy should be initiated utilizing a furosemide stress test (FST).
-
- An FST is considered unresponsive if 1.0-1.5 mg/kg of furosemide results in less than 200mL of urine after two hours.2
- As an alternative to an FST, plasma and urine biomarkers such as neutrophil gelatinase-associated lipcalin (pNGAL/uNGAL), urine ⍺1-microglobulin, urine γ-glutamyl transferase, cystatin C, C terminal fragment of pro-arginine vasopressin, and proadrenomedullin can be measured.
3. If an oliguric patient remains hemodynamically stable but is unresponsive to an FST, renal replacement therapy (RRT) may then be necessary to prevent renal injury and manage electrolytes.
-
- Supportive care should be continued and alternative etiologies should be considered.
- Signs of volume overload such as the presence of peripheral edema, elevated central venous pressure, and increased inferior vena cava diameter on ultrasound should be evaluated.
4. All nephrotoxic medications should be discontinued and the dosing of all drugs excreted by the kidneys should be adjusted to be in accordance with the degree of injury.
Oliguric patients admitted to the hospitals typically have existing comorbidities that contribute to a worse prognosis than that of nonhospitalized patients.5 Low urine output may be secondary to a normal physiological response or due to an underlying pathology but, regardless of the context, oliguria should be promptly evaluated.
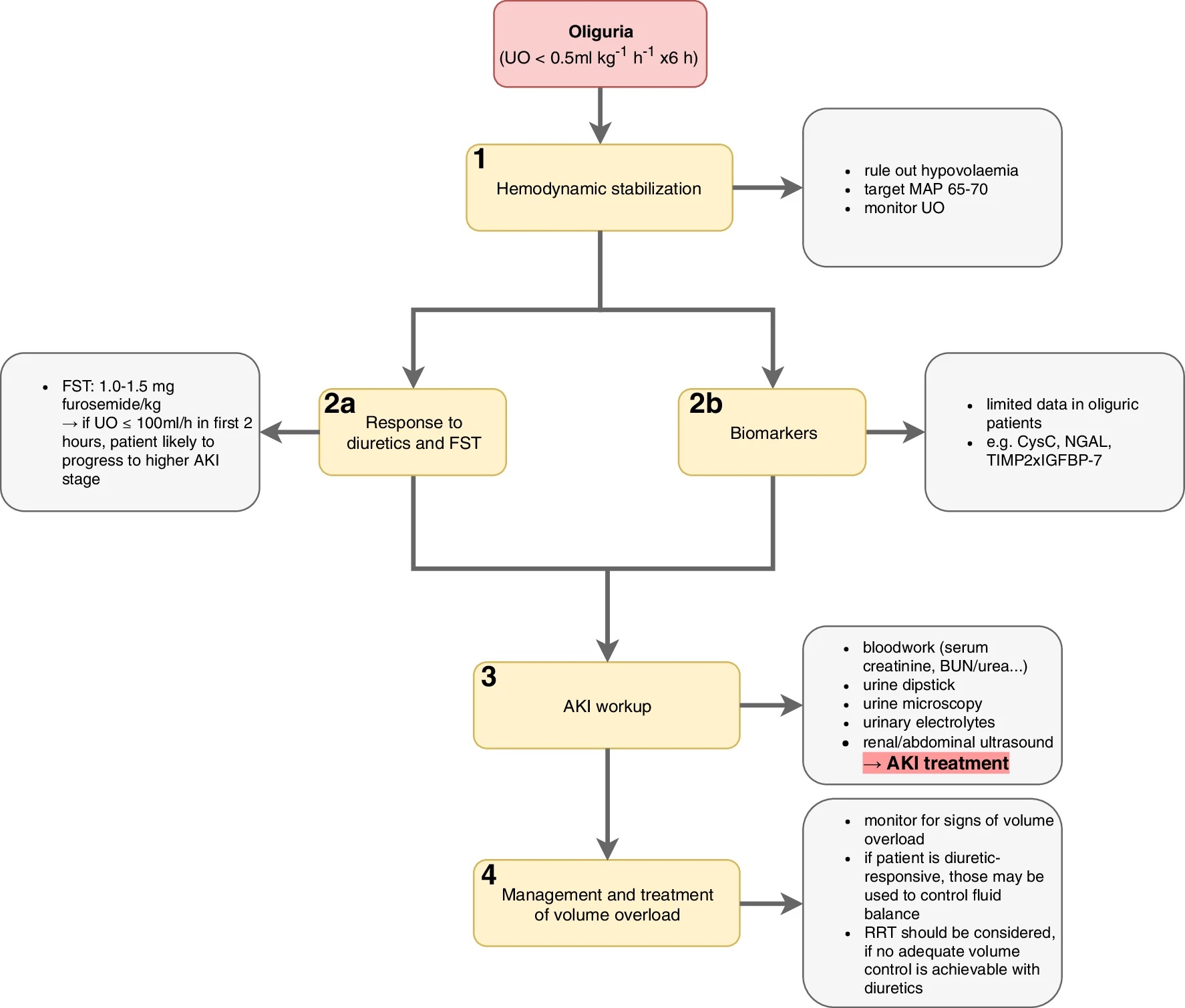
Figure 2. Approach to management of the oliguric patient: Step 1 – hemodynamic stabilization, Step 2a – Response to diuretics and FST, Step 2b – biomarker evaluation, Step 3 – AKI workup, Step 4 – management and treatment of volume overload. UO: urine output, MAP: mean arterial pressure, FST: furosemide stress test, AKI: acute kidney injury, CysC: cystatin C, NGAL: neutrophil gelatinase-associated lipocalin, TIMP-2 x IGFBP-7: tissue inhibitor of metalloproteinase 2 x insulin-like growth factor binding protein 7, BUN: blood urea nitrogen, RRT: renal replacement therapy. Source: Klein SJ, et al. Oliguria in critically ill patients: a narrative review. J Nephrol. 2018;31(6):855-62.3 CC-BY- 4.0.
References
- Klahr S, Miller SB. Acute oliguria. N Engl J Med. 1998;338(10):671-75. PubMed
- Rewa OG, Bagshaw SM, Wang X, et al. The furosemide stress test for prediction of worsening acute kidney injury in critically ill patients: A multicenter, prospective, observational study. J Crit Care. 2019; 52: 109-14. PubMed
- Klein SJ, Lehner GF, Forni LG, Joannidis M. Oliguria in critically ill patients: a narrative review. J Nephrol. 2018;31(6):855-62. PubMed
- Joannidis M, Druml W, Forni LG, et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017: Expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med. 2017;43(6):730-49. PubMed
- Macedo E, Malhotra R, Bouchard J, et al. Oliguria is an early predictor of higher mortality in critically ill patients. Kidney Int. 2011;80(7):760-7. PubMed
- Zarbock A, Koyner J, Hoste E, et al. Update on perioperative acute kidney injury. Anesth Analg. 2018; 127(5): 1236-45. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.