Copy link
Nitric Oxide
Last updated: 09/23/2025
Key Points
- Nitric oxide (NO) is a naturally occurring molecule that is administered via the inhalational route to treat hypoxic respiratory failure through its effects of vascular smooth muscle relaxation and vasodilation in the pulmonary vasculature.
- The only FDA-approved indication for inhaled NO is persistent pulmonary hypertension of the newborn. It is used off-label in several clinical settings, including acute respiratory distress syndrome (ARDS), severe COVID-19 ARDS, and in cardiac surgery.
- Inhaled NO (iNO) exhibits dose-dependent adverse effects, including pulmonary toxicity and methemoglobinemia. Patients receiving NO should be appropriately monitored and weaned to prevent adverse effects.
Introduction
- NO is a colorless, odorless gas that is poorly soluble in water.1
- NO is found naturally, with atmospheric concentrations ranging from 10 to 50 parts per billion.1
- Environmental pollutants such as cigarette smoke and smog can increase the concentration of NO up to 1.7 parts per million (ppm) in highly polluted areas.1
- NO is highly unstable and will undergo oxidation to toxic nitrogen compounds; thus, until the 1990s, scientists viewed NO as a toxic environmental gas.
- In the late 1980s, it was discovered that NO is a key biomolecule involved in maintaining multiorgan homeostasis and perfusion in humans. It is used today in various clinical situations.
- The following summary will review the mechanism of action, physiologic effects, and discuss the wide range of clinical scenarios in which NO has a therapeutic role.
Mechanism of Action
- NO synthase (NOS) combines oxygen and L-arginine to produce NO. Endothelial cells in healthy vasculature secrete NO as part of basic homeostasis and in response to stress or injury. Thus, NO plays an important role in controlling organ perfusion in healthy and disease states.1,2,3 Additionally, some studies have demonstrated NO effects on platelet aggregation and immunomodulation.2,3
- NO produces a vasodilatory effect on the vasculature through the activation of guanylyl cyclase, which metabolizes cyclic guanosine triphosphate to cyclic guanosine monophosphate (cGMP). cGMP dephosphorylates myosin light chains, producing an overall effect of smooth muscle relaxation1 (Figure 1).
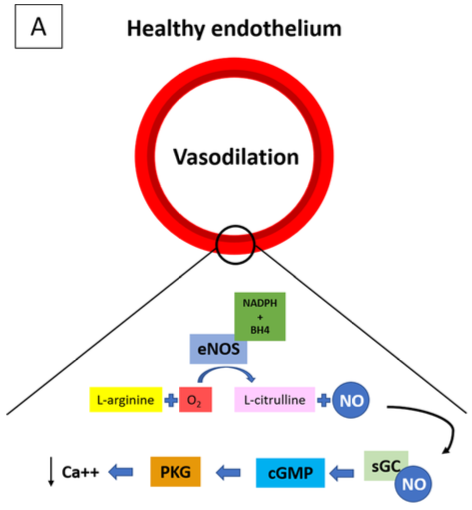
Figure 1. Endogenous NO is produced by NOS by the oxidation of L-arginine to L-citrulline + NO. NO is one of the end-products of this reaction. Most of the cardiovascular effects of NOS are mediated by the activation of sGC, which catalyzes the formation of the second messenger cGMP from GTP. The activation of GMP-dependent PKG leads to vasodilation. Source: Signori D, et al. Inhaled nitric oxide: role in the pathophysiology of cardio-cerebrovascular and respiratory diseases. Intensive Care Med Exp. 2022.1 CC BY 4.0.
Abbreviations: NO, nitric oxide; NOS, NO synthase; sGC, soluble guanylate cyclase; cGMP, cyclic guanosine monophosphate; GTP, guanosine-5’-triphosphate; GMP, guanosine monophosphate; PKG, protein kinase G
- NO also protects the endothelium from platelet aggregation and leukocyte binding.2 Inhaled NO has been shown in healthy subjects to reduce fibrinogen binding, decrease platelet aggregation, and prolong bleeding time through other signaling pathways.2
- NO may exhibit immunomodulatory effects by inhibiting the inflammatory response and leukocyte adhesion.3
- Several medications utilize NO—either directly or indirectly—to exert their clinical effects, primarily through vasodilation or smooth muscle relaxation. These drugs fall into four main categories1:
- Direct iNO
- NO gas diffuses into the pulmonary vasculature -> activates guanylyl cyclase -> increases cGMP -> selective pulmonary vasodilation
- iNO exerts its effects locally on the pulmonary vasculature with minimal systemic vasodilation.
- Nitrovasodilators (e.g., nitroglycerin, isosorbide, and sodium nitroprusside)1
- All nitrovasodilators produce NO -> increase cGMP -> systemic smooth muscle relaxation.
- NO prodrugs provide exogenous NO to the body.
- Phosphodiesterase-5 (PDE-5) inhibitors (e.g., sildenafil, tadalafil)1
- These drugs enhance NO signaling by preventing cGMP breakdown.
- PDE-5 medications require adequate endogenous NO production to effectively work.
- Amino acid precursors (e.g., L-arginine and L-citrulline)1
- Amino acid precursors require adequate endogenous NO production to effectively work.
- Direct iNO
Indications and Contraindications
Indications
- The only FDA-approved use of iNO is for persistent pulmonary hypertension of the newborn (PPHN) in ≥35 weeks gestation.4
- In neonates with oxygenation index (OI) greater than 25, iNO reduced the incidence of death or extracorporeal membrane oxygenation (ECMO).
- iNO did not improve mortality for neonates with congenital diaphragmatic hernia or with OI less than 25.4
- Acute vasodilatory testing with iNO during right heart catheterization is used to find patients with pulmonary artery hypertension (PAH) who have reversible pulmonary vasoconstriction.1
- iNO is the preferred agent for vasodilatory testing because of its rapid onset, selective effect on pulmonary vasculature and short half-life.1
- Vasoreactivity testing identifies a minority of patients in whom PAH is due to increased pulmonary vascular tone as opposed to pulmonary vascular remodeling. These patients have better prognosis and respond well to treatment with high-dose calcium channel blockers.
- iNO is used off-label in several clinical settings:
- To reduce pulmonary vascular resistance and right ventricular afterload after congenital or adult cardiac surgery.5
- As a rescue therapy to improve PaO2 in adults and children with ARDS with or without underlying PAH.
- iNO has been shown to transiently increase PaO2 without a clear mortality benefit. It is often used as a short-term stabilizer until ECMO or recovery.6
Contraindications
- In patients with left heart failure, NO administration may increase pulmonary capillary wedge pressure (PCWP), worsen left ventricular dysfunction and cause pulmonary edema and systemic hypotension.1
- Neonates with congenital heart disease who are dependent on intracardiac mixing, right-to-left shunting, or ductal-dependent systemic blood flow.1
Dosage and Pharmacokinetics
Pharmacokinetics of iNO
- Onset of action: Rapid, dose-dependent, but generally within minutes
- Half-Life: 2-6 seconds
- Metabolism: NO diffuses rapidly across cell membranes and is taken up almost immediately by red blood cells, where it reacts with hemoglobin.2,3
- In oxygenated hemoglobin, NO binds to heme iron to form methemoglobin, nitrite and nitrate.
- In deoxygenated hemoglobin, it forms nitrosyl hemoglobin.
- NO interacts with water to produce nitrite and nitrogen dioxide.
- The end products of NO that enter the systemic circulation are predominantly methemoglobin and nitrate.
- About 70% of nitrate is primarily eliminated by the kidneys.3
Dosing and Administration
- The FDA-approved iNO dose for neonates with PPHN is 20 ppm, administered for a maximum of 14 days or until underlying desaturation has resolved.4
- With increased doses of iNO, a dose-dependent increase in byproducts of metabolism occurs, including toxic nitrogen compounds and methemoglobin.1
- iNO at 40 ppm is used short-term as a rescue dose for severe hypoxemia or pulmonary hypertensive crisis but should be tapered quickly to minimize toxicity.6
Discontinuation of Therapy
- iNO should be weaned in staged decrements, generally with 50% reduction every 2-4 hours. Consider weaning by 1ppm once reach 5ppm.4
Effects on Organ Systems
Central Nervous System
- NO increases cerebral blood flow in response to hypoxia, hypercapnia, increased neuronal activity, and ischemia.
- It exerts both neuroprotective and neurotoxic effects after a cerebral insult.1
- Overproduction of NO can lead to the release of free radicals, oxidative damage and increased permeability of the blood brain barrier.
- Animal models show that systemic NO donors may have a role in preventing delayed cerebral ischemia in subarachnoid hemorrhage.1
Respiratory System
- NO decreases pulmonary vascular resistance. It relaxes the smooth muscle of the pulmonary vasculature, leading to vasodilation.
- iNO preferentially affects well-ventilated lung tissue and diverts blood flow toward ventilated alveoli. This improves ventilation-perfusion matching and arterial oxygenation and reduces pulmonary shunting1 (Figure 2).
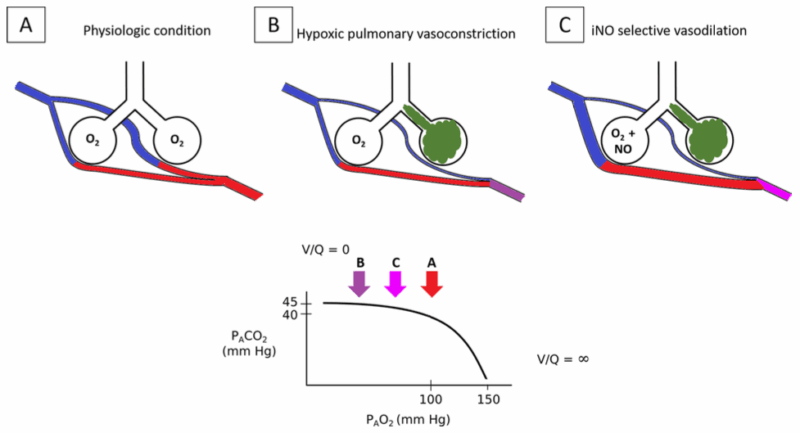
Figure 2. iNO reversal of hypoxemia and pulmonary hypertension during HPV. A: In normal physiological conditions, most of the alveoli are well ventilated and perfused. B: If some of the alveoli are poorly or not ventilated (e.g., atelectasis, pneumonia), the pulmonary capillaries that perfuse these alveoli constrict because of HPV. This increased PVR and limits the blood perfusion of the poorly/not ventilated lungs, thereby limiting V/Q mismatch and pulmonary shunt. C: The administration of NO in the presence of HPV increases the vasodilation of pulmonary vessels that are normally ventilated. This reduces PVR and reverses hypoxemia by diverting the blood flow to ventilated areas. Source: Signori D, et al. Inhaled NO: role in the pathophysiology of cardio-cerebrovascular and respiratory diseases. Intensive Care Med Exp. 2022. CC BY 4.0 Abbreviations: NO, nitric oxide; iNO, inhaled nitric oxygen; HPV, hypoxic pulmonary vasoconstriction; PVR, pulmonary vascular resistance
Cardiovascular System
- NO has a biphasic effect on inotropy.
- At low concentrations, NO has a positive inotropic effect.
- Higher concentrations of NO are associated with a negative inotropic effect, possibly due to its effect on calcium channels in cardiac myocytes.1
- NO reduces myocardial oxygen consumption.1
Hematologic System
- NO inhibits platelet activation and aggregation by inhibiting signaling pathways and platelet recruitment.1
- NO decreases the inflammatory response by inhibiting leukocyte rolling and adhesion to the vascular endothelium. It also inhibits the activity of nicotinamide adenine dinucleotide phosphate oxidase, an enzyme that generates superoxide radicals.1
- NO can cause methemoglobinemia, which decreases oxygen delivery to tissues.
Side Effects and Toxicity
Formation of Toxic Metabolites
- iNO can react with oxygen in the lungs to form nitrogen dioxide, which is a pulmonary irritant.7
- iNO interacts with the superoxide anion to form peroxynitrite, which is cytotoxic and causes extensive cell damage.
- Peroxynitrite formation causes DNA damage and surfactant dysfunction.7
- Prolonged iNO therapy may increase the risk of renal dysfunction in patients with ARDS thought secondary to cytotoxic byproducts of iNO.8
- Prolonged high dose of iNO is associated with methemoglobinemia formation.7
Rebound Pulmonary Hypertension
- Abrupt discontinuation of iNO therapy may result in rebound pulmonary hypertension and worsening hypoxemia.7
References
- Signori D, Magliocca A, Hayashida K, et al. Inhaled nitric oxide: role in the pathophysiology of cardio-cerebrovascular and respiratory diseases. Int Care Med Exp. 2022; 10(1):28. PubMed
- Gries A, Herr A, Motsch J, et al. Randomized, placebo-controlled, blinded and cross-matched study on the antiplatelet effect of inhaled nitric oxide in healthy volunteers. Thromb Haemost. 2000;83(2):309-15. PubMed
- Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907-16. PubMed
- Roberts JD Jr, Fineman JR, Morin FC 3rd, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N Engl J Med. 1997;336(9):605-10. PubMed
- Benedetto M, Piccone G, Gottin L,et al. Inhaled pulmonary vasodilators for the treatment of right ventricular failure in cardio-thoracic surgery: Is one better than the others? J Clin Med. 2024;13(2):564. PubMed
- Adhikari NKJ, Burns KEA, Friedrich JO, et al. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007;334(7597):779. PubMed
- Weinberger B, Laskin DL, Heck DE, Laskin JD. The toxicology of inhaled nitric oxide. Toxicol Sci. 2001;59(1):5-16. PubMed
- Ruan SY, Huang TM, Wu HY, et al. Inhaled nitric oxide therapy and risk of renal dysfunction: a systematic review and meta-analysis of randomized trials. Crit Care. 2015;19(1):137. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.