Copy link
Epidural and Combined Spinal-Epidural: Pharmacology, Technique, and Side Effects
Last updated: 12/19/2023
Key Points
- Spinal anesthesia is achieved through the introduction of local anesthetic (LA) to the lumbar cerebral spinal fluid (CSF), which diffuses down a concentration gradient across axonal cell membranes, binding to voltage-gated sodium channels on the cytoplasmic surface, triggering autonomic, sensory, and motor blockade. Epidural anesthesia relies on blockade of nerve roots, as well as some diffusion across the dura.
- Appropriate monitoring during the procedure includes all basic vital signs, and anatomical landmarks are generally reliable in identifying vertebral levels, with point-of-care neuraxial ultrasonography being helpful in some cases.
- Spread of anesthesia can be affected by the addition of adjuvants, level and duration of blockade, volume and concentration of LA, and patient positioning.
Pharmacology of Local Anesthetics
- Bupivacaine, ropivacaine, mepivacaine, 2-chloroprocaine are commonly used LA for spinal anesthesia. Epidural anesthesia most frequently relies on bupivacaine, though lidocaine and 2-chloroprocaine are also used, especially for labor epidurals.
- The metabolism of the LA depends on local blood flow, degree of protein binding, lipophilicity, LA type, and adjuvants.
- Once the LA is introduced to the lumbar CSF, the nonionized molecule diffuses down a concentration gradient across the axonal cell membranes, binding to voltage-gated sodium channels on the cytoplasmic surface triggering autonomic, sensory, and motor blockade several dermatomal levels above and below the site of injection.1
Since nerve roots are not covered by dura mater, the LA solution is rapidly taken up by nerve roots and rootlets. Agents that get absorbed into the CSF will diffuse across the pia mater into the spinal cord, concentrating primarily in the dorsal column and the anterior and lateral spinothalamic tracts.1 - Metabolism of LA occurs via vascular uptake of agent and depends on local blood flow, degree of protein binding, lipophilicity, LA type, and adjuvants. Amide local anesthetics are metabolized in the liver, and ester local anesthetics by plasma cholinesterase.1
Adjuvants
Opioids
- Intrathecal and epidural opioids are commonly administered alongside LA agents to achieve synergistic analgesia and anesthesia.
- Opioids potentiate neuraxial anesthesia by binding to the mu-opioid receptors located in lamina II (the substantia gelatinosa) as well as I and V, located in the dorsal horn of the spinal cord.
- Long-acting hydrophilic opioids (preservative-free morphine or less commonly diacetylmorphine) provide long-lasting analgesia due to their persistence in the CSF, but can cause side effects such as sedation, pruritus, nausea and vomiting, urinary retention, and hypoventilation.
- Short-acting opioids (such as fentanyl and sufentanil) are more lipophilic and can cause signs of systemic absorption within minutes of injection and are commonly added to patient-controlled epidural anesthesia infusions for labor or postoperative pain.
- See the OA Summary on neuraxial opioids for more details. Link
Epinephrine
- Preservative-free epinephrine is commonly added to both intrathecal and epidural LA solutions at a concentration of 1:200,000, i.e., 5mcg/mL.
- Epinephrine both potentiates density of block and speeds the onset of neuraxial blockade, prolongs the duration of short-acting LA, and acts as a marker of inadvertent intravascular injection.1,3
- Epinephrine may be weakly neurotoxic, and the effect may be additive with the weak neurotoxicity of LA, and there is a risk of drug error when diluting the solution.
Alpha-2 Agonists
- Clonidine and dexmedetomidine may be added to both intrathecal and epidural LA solutions, potentiating and prolonging neuraxial blockade with alpha-2 agonism at the spinal cord and dorsal root ganglions.
- Epidural alpha-2 agonists may speed onset time and increase block density, without affecting dermatomal spread.1,3
- Sedation and lingering hypotension are the most common side effects. Prolonged hypotension in some cases is an important limitation, particularly with the less alpha-2-selective clonidine.
- See the OA Summary on epidural clonidine and dexmedetomidine for more details. Link
Bicarbonate
- Alkalinization of LA solutions leverages the pharmacokinetic properties of LA by favoring the nonionized form of LA molecule, speeding its passage across the lipid bilayer and therefore hastens the onset of sodium channel blockade.
- Addition of 1mL of preservative-free 8.4% bicarbonate solution per 9 mL of LA solution modestly reduces onset time of epidural block1,3
- Risks include precipitation of the solution (reported with ropivacaine) and inadvertent use of preservative-containing bicarbonate.
Technique
Setup and Positioning
- Proper positioning is imperative to success. Exaggerated lumbar flexion, tilt, or pelvic flexion can tighten the interspinous spaces and make placement much more difficult.
- Appropriate monitoring should include standard American Society of Anesthesiologists monitors: noninvasive blood pressure, electrocardiography, pulse oximetry, and capnography if performed under sedation.
- The seated position with an exaggerated kyphosis offers the best first-pass success rates, although certain clinical requirements may necessitate lateral positioning (e.g. patients with hip fractures).
- A pillow under the patient’s knees or placing the patient in the lateral position can help optimize placement of the epidural when lumbar lordosis is nonoptimal.
- Anatomical landmarks are generally reliable in identifying vertebral levels.
- Point-of-care neuraxial ultrasonography is helpful in identifying the ideal level, midline, and depth to the epidural space in some cases.
Epidural Technique
- The midline approach is typically considered the easiest, while the paramedian approach is well-suited to narrow interspaces or difficulty with flexion. However, a midline approach higher in the thoracic spine can become more challenging or not feasible, as the spinous processes in the thoracic spine become longer and may obscure the interspinous space.2
- After anesthetizing the skin with 2% lidocaine, a Tuohy needle is introduced either midline or paramedian and driven towards the epidural space. Loss of resistance to air or saline, in a continuous or intermittent fashion, is utilized to identify when the needle tip has successfully entered the epidural space.
- The catheter should thread easily without resistance. The catheter is then tested for inadvertent intrathecal (within the subarachnoid space) or intravascular placement. Aspiration should be negative for heme and a test dose with 3mL of 1-2% lidocaine with 1:200,000 epinephrine should not produce spinal effects or increased heart rate. The needle is then withdrawn, and the catheter secured.2
Combined Spinal-Epidural (CSE) Technique
- For CSE, the placement should begin at the line drawn from the top of the iliac crest is known as “Tuffier’s” line. This line generally crosses the body of L4 or the L4-L5 interspace. The conus medullaris, the terminal segment of the spinal cord, may extend down to L3 in 2% of adults. Therefore, palpating the top of the iliac crests helps locate an ideal space for spinal needle entry.2
- After placement of the Tuohy in the epidural space as directed above, a small gauge needle is thread through the Tuohy (known as the “needle through needle” technique) and past the dura into the CSF. Once CSF is flowing from the needle, the medication is injected, with or without mixing of withdrawn CSF in a technique known as barbotage. The pencil-point needle is then withdrawn through the Tuohy and the catheter threaded as above. Aspiration and test-dose should be performed to confirm correct catheter placement. The patient should then be assisted into a flat position.
- Pencil-point needles are associated with a lower risk of postdural puncture headaches, as are thin gauges (frequently 25 gauge or 27 gauge).
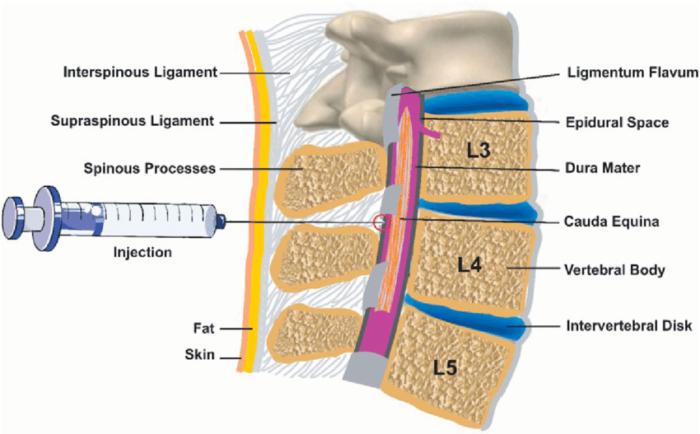
Figure 1. Anatomy of the epidural space. Source: Wikipedia. Leila Kafshdooz, et al. CC BY 4.0. Link.
Spread
- Spread of medications administered via an epidural depends on different factors than those administered via a spinal directly into CSF3:
- LA properties: volume appears to have the greatest impact on spread of medications given through an epidural (compared with total dose and baricity as seen with spinals). Generally, each dermatome above and below the location of the epidural can be covered incrementally by 1-2mL of LA per desired level. Concentration affects the density of block, i.e., whether sensory block is analgesic vs anesthetic and degree of motor block.
- Patient factors: whereas CSF volume correlates with medication spread with spinals, epidural spread correlates more with the size of the epidural space. Obesity, pregnancy, and older age are associated with tighter epidural spaces and therefore increased spread. Elderly patients in particular may be more susceptible to the autonomic side effects of epidurals. There is some evidence that lateral positioning may favor spread of medication to the dependent side, though this can be overcome with enough volume.
- Technical factors: because the thoracic spine is much longer than the lumbar spine, choice of interspace level for thoracic epidurals affects spread more than with lumbar epidurals. Tuohy bevel orientation (cranial vs caudad vs lateral) does not appear to affect spread but may affect incidence of one-sided epidurals or paresthesias during placement. Faster injection of LA appears to quicken the onset of anesthesia, but whether speed of injection affects spread remains controversial.
- Epidurals can typically have up to 6 dermatomal levels of spread, and this is primarily determined by volume of LA and flow rates.
- Spread can also be affected by the addition of vasoconstrictors (e.g., 0.1 – 0.2 mg of epinephrine, or 0.2 to 0.5 cc of 1:1000 epinephrine). Epinephrine may have the additional benefit of some alpha-2 related analgesia.
Side Effects and Complications
- Hemodynamic changes are common after spinal anesthesia, with decreases in systemic vascular resistance and preload. Epidurals have fewer profound effects overall than CSEs. Hypotension can be treated with a modest reverse Trendelenburg position and hydration, with ephedrine as the first-line drug.
- Postdural puncture headaches (PDPH) can occur from inadvertent puncture of the dura with the relatively large-bore Tuohy needle, or even with the intended puncture of dura using the spinal needle. For the latter, PDPH risk can be reduced with small gauge pencil point needles. Risk of PDPH ranges between 1-2% for most patients receiving neuraxial anesthesia, and generally declines with increasing age.4 Consensus guidelines exist for PDPH management, including conservative management and indications/contraindications/management of epidural blood patches.4
- Paresthesias may occur with either dural puncture with the spinal needle or when threading the epidural catheter, usually due to contact with a nerve root.2 If the paresthesias do not resolve quickly, the needle should be withdrawn, and the procedure reattempted at a different level.
- High spinal anesthesia occurs when the spread of the local anesthetic affects spinal nerves above T4, and it can result in cardiovascular and/or respiratory compromise. LA flow even higher up into the cranium can result in loss of consciousness and seizures. This would require airway control and ventilation, intravenous fluids, and sympathomimetics. High spinals during CSEs may occur due to inadvertent intrathecal catheter placement, or from large volumes of epidural LA that may enter the intrathecal space via the dural puncture created by the spinal needle.2
- Other potential side effects include failed epidural, urinary retention, backache, subdural injection, and hypoventilation.
- Subdural (between epidural space and subarachnoid space) injection is a rare but possible complication, and difficult to diagnose.5
- Due to the anatomy of the subdural space, it most often presents as profound sensory block with minimal motor block or sympathetic block. The level of block is out of proportion to the amount of LA injected for an epidural catheter, and highly variable e.g., more likely to be one-sided or miss some sensory levels.
- However, because the space is narrow and extends craniocaudally, LA injected here can quickly reach the intracranial subdural space and lead to depressed mental status, respiratory depression/apnea from direct respiratory drive suppression, and hypotension.
- Management of suspected subdural injection includes supportive care and immediate discontinuation of the catheter.
- The most serious complications include bleeding, infection (including epidural abscess, and less commonly meningitis) and nerve damage, all of which are very rare.2
References
- Cook B, Doyle E. The use of additives to local anaesthetic solutions for caudal epidural blockade. Paediatr Anaesth. 1996;6(5):353-9. PubMed
- Cook TM. Combined spinal-epidural techniques. Anaesthesia. 2000;55(1):42-64. PubMed
- Visser WA, Lee RA, Gielen MJ. Factors affecting the distribution of neural blockade by local anesthetics in epidural anesthesia and a comparison of lumbar versus thoracic epidural anesthesia. Anesth Analg. 2008;107(2):708-21. PubMed
- DelPizzo K, Luu T, Fields KG, et al. Risk of postdural puncture headache in adolescents and adults. Anesth Analg. 2020;131(1):273-9. PubMed
- Agarwal D, Mohta M, Tyagi A, et. al. Subdural block and the anaesthetist. Anaesth Intensive Care. 2010;38(1):20-6. PubMed
Other References
- Toledano R, Van de Velde M. Epidural Anesthesia and Analgesia. New York School of Regional Anesthesia. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.