Copy link
Mitochondrial Encephalomyopathy with Lactic Acidosis and Stroke-Like Episodes Syndrome
Last updated: 08/11/2025
Key Points
- Mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS) syndrome is a mitochondrial disorder that typically presents between the ages of 2 and 10 years after a period of normal early psychomotor development. However, infantile and adult-onset cases have been documented.
- The condition is believed to stem from mutations in mitochondrial proteins, resulting in multisystem dysfunction including cardiomyopathy, encephalopathy, seizures, and cerebellar ataxia.
- The main anesthetic considerations in patients with MELAS syndrome stem from the concomitant depressant effects of anesthetics on mitochondrial function.
Introduction
- MELAS syndrome is a maternally inherited mitochondrial disorder that typically presents after a normal developmental period. The common clinical manifestations include characteristic stroke-like episodes, seizures, recurrent headaches, vomiting, and hearing loss.
- The disease typically follows a relapsing-remitting pattern, ultimately leading to progressive neurologic decline and dementia.1
- This summary provides an overview of MELAS syndrome, including its clinical manifestations, proposed pathophysiology, and key anesthetic considerations in the management of affected individuals.
- Mutations in mitochondrial transfer RNA contribute to the development of MELAS. The A3243G (m.3243 A > G) variant encompasses 80% of cases, while the T3271C variant accounts for about 10%.1
- The estimated incidence of MELAS is 1 in 4,000.2
Pathophysiology
- MELAS syndrome exhibits heteroplasmy, leading to varying proportions of mutated mitochondrial DNA across the various organ systems of one individual.
- Two main theories are proposed regarding the underlying pathophysiology of MELAS syndrome:
- Cytopathic theory: Abnormal oxidative phosphorylation leads to neuronal dysfunction and cell death during periods of high metabolic activity.2
- Angiopathic theory: Abnormal mitochondria in the endothelial and smooth muscle cells of the vasculature cause impaired vasodilation and perfusion.
- Tissues with the highest levels of metabolic activity are primarily affected.
- Increased levels of anaerobic metabolism occur due to dysfunction of the mitochondrial respiratory chain and oxidative phosphorylation, which results in increased levels of lactic acid during acute episodes.
- Free radical production induces vasoconstriction, which in turn disrupts the equilibrium with vasodilatory substances such as nitric oxide.
- Stroke-Like episodes
- A hallmark feature of MELAS syndrome is the occurrence of stroke-like episodes, which differ from typical ischemic strokes in several ways.
- Symptoms often do not align with typical vascular territories.
- Frequent fluctuations in MRI signals occur (Figure 1).
- The clinical, imaging, electroencephalographic, and neuropathological features observed during stroke-like episodes in MELAS closely resemble those seen in epilepsy.1
- Studies have proposed a link between these episodes and a deficiency of nitric oxide, potentially resulting from reduced plasma concentrations of its precursors, L-arginine or citrulline.3
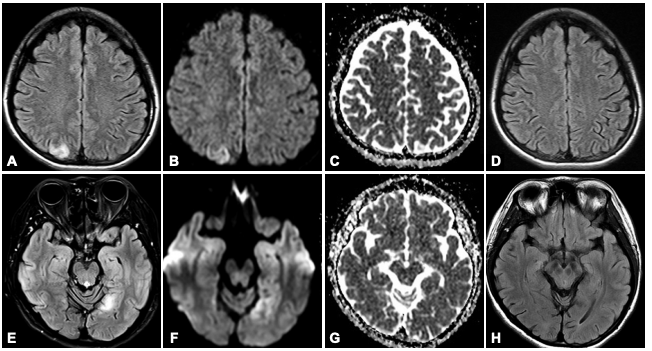
Figure 1. Magnetic resonance imaging using diffusion-weighted imaging and fluid-attenuated inversion recovery sequences demonstrated a right parietal lobe lesion during the initial episode (A–C) and a left occipital lobe lesion during the second episode (E–G). Follow-up imaging revealed resolution of both abnormalities (D, H).4
Source: Jung I, Park SH, Kim DW. Diffusion-weighted and FLAIR MRI scans showing reversible stroke‑like lesions in *Mitochondrial encephalopathy, lactic acidosis, and stroke‑like episode syndrome presenting with prolonged visual aura. Journal of Clinical Neurology. 2015;11(1):104–105. Licensed under CC BY‑NC 3.0 via PubMed Central
Clinical Presentation
- Stroke-like episodes are a cardinal manifestation of MELAS Syndrome. These episodes can consist of symptoms such as hemiparesis, hemianopia, and cortical blindness.
- However, migraines or migraine-like headaches can be the only presentation of stroke-like episodes in certain patients.2
- Neurologic:
- Focal or generalized seizures
- Sensorineural hearing loss
- Peripheral neuropathy
- Vision loss due to optic nerve atrophy
- Night blindness due to pigmentary retinopathy
- Autism spectrum disorders
- Progressive cognitive decline
- Alzheimer dementia (specifically associated with the m.3242 A > G mutation)2
- Cardiovascular:
- Conduction abnormalities (e.g., Wolff-Parkinson-White Syndrome)
- Cardiomyopathy
- Endocrine/Metabolic
- Failure to thrive
- Polydipsia and polyuria (often reflecting the development of diabetes mellitus or other endocrinopathies)
- Gastrointestinal
- Recurrent vomiting
- Feeding intolerance
- Musculoskeletal:
- Proximal muscle weakness
- Exercise intolerance
- Psychiatric:
- Proposed associations with bipolar disorder and major depressive disorder
Anesthetic Considerations
Preoperative Considerations
- The patient’s current treatment regimen for MELAS syndrome should be maintained as long as possible.
- Excess metabolic stressors, such as prolonged fasting, dehydration, hypothermia, hypovolemia, acidosis, and hypoglycemia, should be avoided.
- Lactate-containing intravenous fluids should be avoided.6
- Regular blood gas analysis should be performed to monitor pH, glucose, and electrolytes.
General Anesthetic Considerations
- Patients with MELAS syndrome undergoing general anesthesia are at risk for respiratory failure, cardiac depression, conduction defects, and dysphagia.7
- Many general and adjunct anesthetic agents have been shown to depress mitochondrial function in vitro; however, clinical tolerability varies.7
- Muscle relaxation should be monitored intraoperatively to avoid excess administration of medication during surgery.6
Volatile Anesthetics
- Volatile anesthetics suppress oxidative phosphorylation, specifically at complex I of the electron transport chain.7
- Rapid elimination allows for the return of mitochondrial function after discontinuation, as opposed to many parenteral anesthetics that are metabolized hepatically.
- The use of central nervous system (CNS) monitoring with processed electroencephalography to prevent excessive dosing of inhaled anesthetics should be considered.7
Parenteral Anesthetics
- Most parenteral anesthetics inhibit complex I.
- Propofol disrupts mitochondrial metabolism via at least four mechanisms, including uncoupling of oxidative phosphorylation at complexes I, II, and IV and inhibition of acylcarnitine transferase, contributing to the risk of propofol infusion syndrome.
- Clinicians should exercise caution with the use of propofol due to the risk of propofol-related infusion syndrome (PRIS), especially in longer surgeries or if higher doses are being used.
- PRIS manifestations include refractory bradycardia, metabolic acidosis, rhabdomyolysis, hyperlipidemia, and liver injury.
- Limited bolus dosing is generally tolerated.
- Clinicians should exercise caution with the use of propofol due to the risk of propofol-related infusion syndrome (PRIS), especially in longer surgeries or if higher doses are being used.
Local Anesthetics
- Regional anesthesia can provide analgesia without the risk of respiratory depression.
Opioids and Adjunct Analgesics
- Opioids can reduce oxygen demand through effective analgesia, but may impair respiratory control in some patients.
- Most opioids have minimal mitochondrial effects apart from morphine.
- Due to its rapid offset and swift metabolism by serum cholinesterases, the ultrashort-acting opioid remifentanil is favored.
- Sedative benzodiazepines and the CNS alpha-adrenergic agonist dexmedetomidine are also generally considered safe.7
- Analgesic doses of ketamine have been well tolerated.7
Choice of Neuromuscular Blocker
- The use of atracurium and nondepolarizing neuromuscular blockers should be considered if relaxation is required.5
- The use of succinylcholine may result in rhabdomyolysis and life-threatening hyperkalemia due to upregulation of nicotinic acetylcholine receptors in skeletal muscle.5
Postoperative Considerations
- Metabolic Monitoring:
- Frequent glucose checks, with the first check within 15 minutes of entering the postanesthesia care unit (PACU)
- Adequate control of postoperative nausea and vomiting
- Regular arterial blood gases, especially to monitor for lactic acidosis8
- Avoidance of prolonged nil per os status; resume feeds or IV dextrose-containing fluids to prevent catabolic stress
- Continued avoidance of lactate-containing IV fluids
- Respiratory Monitoring:
- Extended monitoring in the PACU or intensive care unit can be warranted for hypoventilation, risk of aspiration, or opioid sensitivity.7
- Cardiac Surveillance
- Telemetry is recommended to monitor for any conduction abnormality, bradyarrhythmia associated with PRIS, or autonomic instability.
- Medication Considerations:
- Home medications should be resumed as soon as possible, including any anticonvulsants and metabolic supplements.
- Valproate should be avoided as it impairs mitochondrial function and can exacerbate encephalopathy.
- Clinicians should be cautious with antiemetics such as metoclopramide, as they may lower the seizure threshold.
- Neurologic Assessment:
- o Clinicians should monitor for new focal deficits as this raises concern for a possible stroke-like episode.
- Seizures or postictal confusion
- Delayed emergence due to anesthetic sensitivity or encephalopathy
References
- O’Ferrall EK, Shefner JM, Dashe JF. Mitochondrial myopathies: Clinical features and diagnosis. In: Post TW, ed. UpToDate; 2025. Accessed July 20, 2025. Link
- Pia S, Lui F. Melas Syndrome. In: StatPearls (Internet). Treasure Island, FL. StatPearls Publishing; 2025. July 20, 2025. Link
- El-Hattab A, Adesina AM, Jones J, et al. MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options. Molecular Genetics and Metabolism. 2015;116(1-2):4-12. Accessed July 21, 2025. Link
- Jung I, Park S, Kim DW. Mitochondrial encephalopathy, lactic acidosis, and stroke-like episode syndrome presenting with prolonged visual aura. J Clin Neurol. 2015; 11 (1): 104-105. PubMed
- Salehpoor MS, Paluska MR, Falcon R, et al. Anesthetic management of a patient with mitochondrial encephalomyopathy, lactic acidosis, and stroke like episodes syndrome during extensive spinal surgery with both motor evoked potentials and somatosensory evoked potentials: A case report. Cureus. 2023;15(10). PubMed
- Kishida T, Ishida Y, Okada T, et al. Successful perioperative management of cochlear implantation in a patient with mitochondrial encephalopathy, lactic acidosis, and stroke like episodes (MELAS). Cureus. 2022;14(8). Link
- Hsieh VC, Krane EJ, Morgan PG, et al. Mitochondrial disease and anesthesia. Mitochondrial disease and anesthesia. Journal of Inborn Errors of Metabolism and Screening. 2017;5(6). Link
- Hoppe K, Plunien R, Lehmann-Horn F, et al. Mitochondrial cytopathy, mitochondrial myopathy, mitochondrial encephalomyopathy. OrphanAnesthesia. Published 2013. Accessed July 21, 2025. Link
Other References
- Ross F, Morgan P. Mitochondrial disease and anesthesia. OA-SPA Ask the Expert Podcast. OpenAnesthesia. Published December 1, 2018. Accessed August 11, 2025. Link
- Kanmanthreddy S. Anesthetic considerations in patients with mitochondrial disease. OA-SPA Pediatric Anesthesia Virtual Grand Rounds. OpenAnesthesia. Published September 1, 2023. Accessed August 11, 2025. Link
- Niezgoda J, Morgan PG. Anesthetic considerations in patients with mitochondrial defects. In: Gregory GA, Andropoulos DB, eds. Gregory’s Pediatric Anesthesia. 5th ed. Wiley-Blackwell; 2012:785-793.
- Davis PJ, Cladis FP, Motoyama EK, eds. Smith’s Anesthesia for Infants and Children. 9th ed. Elsevier; 2017:850 880. Chapter: Inherited Metabolic Disease.
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.