Copy link
Metoclopramide
Last updated: 07/08/2025
Key Points
- Metoclopramide has both prokinetic and antiemetic effects, acting centrally via dopamine D2 receptor antagonism at the chemoreceptor trigger zone (CTZ) and peripherally via 5-HT4 receptor agonism to enhance upper gastrointestinal (GI) motility.
- Metoclopramide has a rapid onset of action when administered intravenously, making it suitable for promoting preoperative gastric emptying and reducing postoperative nausea and vomiting.
- Major adverse effects include the risk of tardive dyskinesia, which is potentially irreversible and increases with cumulative dose and duration, especially in elderly women, diabetics, and those on antipsychotics; other notable side effects are acute dystonic reactions, sedation, and extrapyramidal symptoms.
Introduction
Figure 1. Chemical Structure of metoclopramide (trade name Reglan). Source: Wikimedia Commons, public domain. Link
Physiochemical Properties1
- Metoclopramide hydrochloride monohydrate (4-amino-5-chloro-N-[2-(diethylamino)ethyl]-2-methoxy benzamide monohydrochloride monohydrate) is a white, odorless, crystalline substance that is freely soluble in water.
- Metoclopramide is available in 5 mg and 10 mg tablets for oral administration and is commonly diluted to 0.2-0.5 mg/mL in compatible solutions (normal saline or dextrose 5%) for intravenous (IV) infusion.
Mechanism of Action
- Metoclopramide achieves antiemetic effects by antagonizing the dopamine D2 receptors in the CTZ in the area postrema of the medulla oblongata.2
- It also elicits prokinetic effects in the upper GI system by agonizing 5-HT4 receptors in the myenteric plexus, thereby enhancing the release of neuronal acetylcholine to promote coordinated contractions and accelerate gastric emptying, independent of vagal input.3
- At higher doses, metoclopramide antagonizes 5-HT3 receptors on vagal afferent nerve terminals and in the CTZ, contributing to its antiemetic properties in a mechanism similar to ondansetron.4
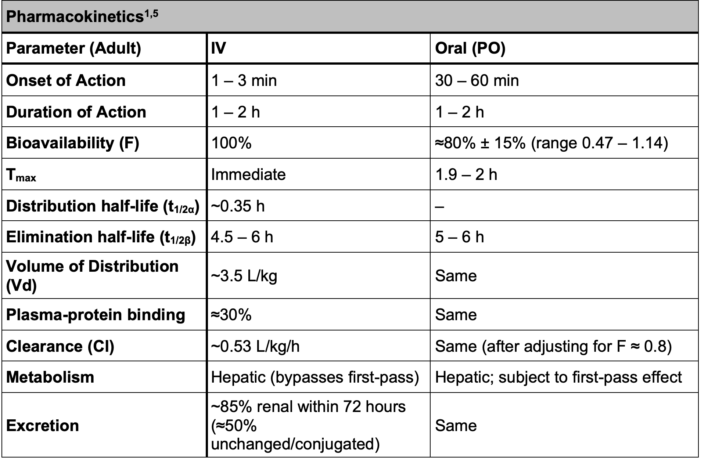
Table 1. Pharmacokinetics of metoclopramide
- Values apply to healthy adults with normal renal function; clearance and t1/2 increase in renal impairment.
- High Vd and short distribution phase reflect extensive tissue uptake and rapid redistribution.
- Differences between IV and oral administration are clinically relevant mainly for onset, bioavailability, first-pass metabolism, and Tmax.
- Linear kinetics observed: plasma levels increase proportionally with dose.
Systemic Effects1
Central Nervous System
- Metoclopramide crosses the blood-brain barrier and antagonizes dopamine D2 receptors in the CTZ.
- It produces dose- and duration-dependent central effects including sedation, restlessness, and alterations in mood or sensorium.
Endocrine System
- Metoclopramide increases serum prolactin levels via hypothalamic D2 blockade, with potential downstream hormonal effects.
- It stimulates transient aldosterone release, which may promote fluid retention in susceptible patients.
Cardiovascular System
- Metoclopramide may affect autonomic tone and cardiac conduction, with potential for blood pressure variability and arrhythmogenic effects.
- It is contraindicated in patients with pheochromocytoma due to risk of hypertensive crisis.
GI System
- Metoclopramide enhances upper GI motility by increasing lower esophageal sphincter tone, gastric contractions, and duodenal/jejunal peristalsis.
- It accelerates gastric emptying, reducing gastric residual volume.
Hepatic and Renal Systems
- Metoclopramide is subject to hepatic metabolism (CYP2D6) and renal elimination; impaired function may prolong drug exposure and effects.
- Rare hepatotoxic effects have been reported in postmarketing surveillance.
Hematologic System
- Metoclopramide rarely alters hematopoiesis, with isolated reports of blood dyscrasias including methemoglobinemia and agranulocytosis.
Other Systems
- Metoclopramide may cause systemic immune and neurologic effects such as hypersensitivity reactions or visual/urinary disturbances.
Clinical Uses
GI Motility Disorders1
- Metoclopramide is FDA-approved for symptomatic treatment of gastroesophageal reflux in adults unresponsive to standard acid suppression therapy.
- It is indicated for short-term management (4–12 weeks) of acute and recurrent diabetic gastroparesis, improving gastric emptying and reducing nausea, bloating, and early satiety.
- Typical oral dosing for GI motility disorders ranges from 10–15 mg orally up to four times daily, 30 minutes before meals and at bedtime.
- The maximum recommended daily dose is 60 mg for gastroesophageal reflux and 40 mg for diabetic gastroparesis.
Antiemetic Therapy
- Metoclopramide is effective in the prevention and treatment of postoperative nausea and vomiting (PONV), which is commonly administered as a 10 mg IV dose within the final 15–30 minutes before the anticipated completion of the surgical procedure or just prior to the discontinuation of anesthesia.6
- Previously considered ineffective due to fraudulent data, metoclopramide was re-evaluated in a 2013 meta-analysis by De Oliveira et al., which excluded discredited studies and found that 10 mg IV significantly reduces PONV at 24 hours compared to placebo (odds ratio 0.58, 95% CI 0.43-0.78; NNT 7.8), reaffirming its value as an antiemetic.6
- High IV doses (1–2 mg/kg) were historically used in combination with glucocorticoids for chemotherapy- and radiation-induced nausea, but is now rarely used in this setting due to limited efficacy compared to other therapies.7
Preoperative Gastric Emptying8
- Metoclopramide significantly reduces gastric volume and increases pH. When administered to healthy patients prior to anesthesia, it lowered gastric fluid volume by 70-80% compared to placebo at matched time points and increased gastric pH by approximately 1.5 units.9
- It is available preoperatively to accelerate gastric emptying and reduce gastric volume in patients with suspected delayed gastric transit (e.g., emergency, pregnancy, diabetes), as well as for facilitation of radiologic or endoscopic procedures.
- It is often dosed at a 10 mg IV dose 10–30 minutes prior to procedures.
- While it is effective in reducing gastric volume and pH, there is insufficient evidence to evaluate its effect on the incidence of pulmonary aspiration.
- Routine use is not recommended except in patients at increased risk for delayed gastric emptying (e.g., patients with idiopathic or postsurgical gastroparesis, long-standing diabetes mellitus, Parkinson’s disease, multiple sclerosis, scleroderma, cystic fibrosis, morbid obesity, or history of upper GI surgery).
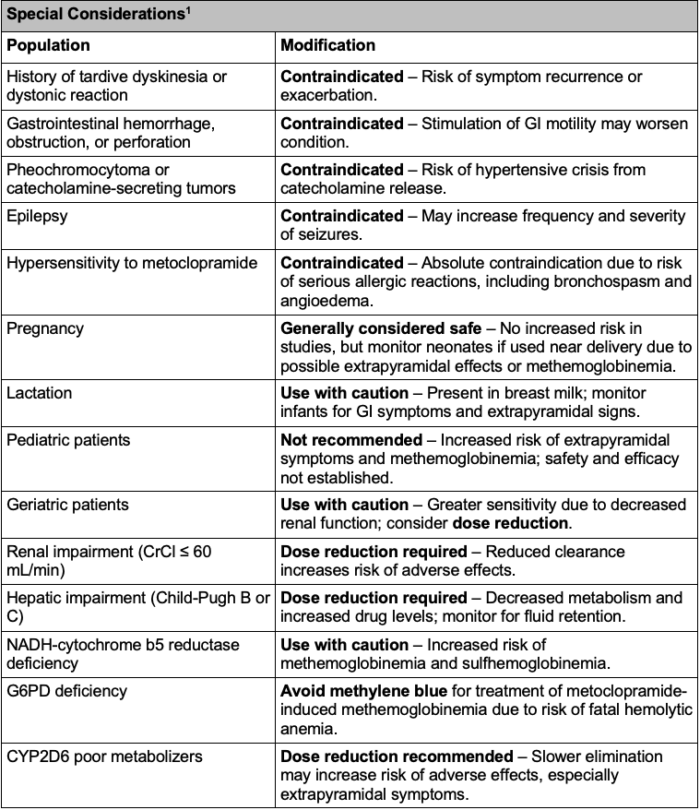
Table 2. Special considerations for metoclopramide use
Side Effects1,10
The risk of serious adverse effects, such as tardive dyskinesia and endocrine disturbances, is primarily associated with prolonged oral use. For short-term IV administration in the perioperative setting, the overall risk is low.
- Tardive dyskinesia is most likely in elderly patients, especially elderly women, and those with diabetes; risk increases with higher cumulative dose and duration of therapy, particularly beyond 12 weeks.
- Other extrapyramidal symptoms (including acute dystonic reactions, parkinsonism, and akathisia) are more common in adults under 30 years of age, at higher-than-recommended doses, and in those with a history of movement disorders or concomitant use of other dopamine antagonists; symptoms can occur within the first 24–48 hours of therapy.
- Neuroleptic malignant syndrome is rare but can occur at any age, with increased risk in those receiving other dopamine antagonists or with underlying neurologic disease.
- Restlessness, drowsiness, fatigue, and lassitude occur in approximately 10% of patients, with a higher risk associated with standard or higher doses (10 mg four times daily) and longer therapy duration.
- Depression (including suicidal ideation), confusion, hallucinations, and convulsive seizures are more likely in patients with a history of psychiatric illness, at higher doses, or with prolonged use.
- Hypertension, hypotension, arrhythmias, and acute congestive heart failure are more likely in patients with pre-existing cardiovascular disease, those with undiagnosed pheochromocytoma, or at higher doses; fluid retention risk is increased in patients with cirrhosis or heart failure.
- Hyperprolactinemia (manifesting as galactorrhea, amenorrhea, gynecomastia, impotence) is more likely with prolonged use and higher doses, and may be of particular concern in patients with a history of hormone-sensitive cancers.
- GI effects (nausea, diarrhea) can occur in any patient, but the risk increases with dose.
- Agranulocytosis, neutropenia, leukopenia, methemoglobinemia, and sulfhemoglobinemia are rare but may occur at any dose, with increased risk in those with underlying hematologic or metabolic disorders.
- Allergic reactions (bronchospasm, urticaria, rash, angioedema) are more likely in patients with a history of asthma or allergies.
- Visual disturbances can occur in any patient, with no specific risk factors identified.
Drug Interactions1
Metoclopramide’s pharmacodynamic profile means that coadministration with other agents acting on histamine, acetylcholine, or dopamine receptors can increase the risk of adverse effects. Clinically relevant interactions are included in Table 3.
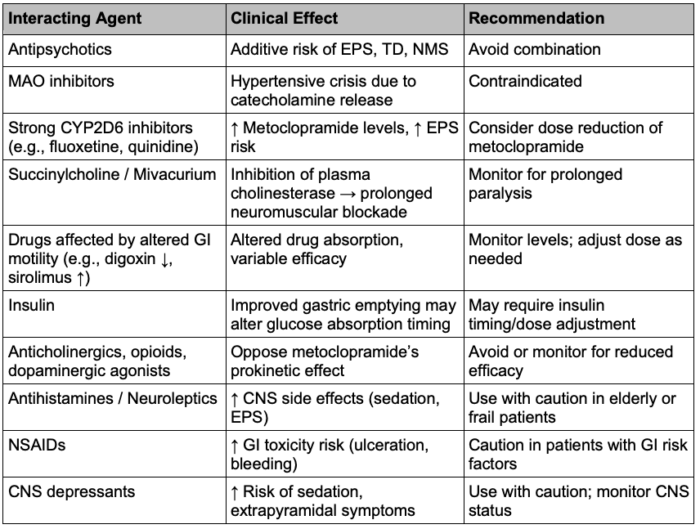
Table 3. Drug interactions of metoclopramide. Abbreviations: EPS = extrapyramidal symptoms, TD = tardive dyskinesia, NMS = neuroleptic malignant syndrome, MAO = monoamine oxidase, GI = gastrointestinal, CNS = central nervous system, NSAIDs = non-steroidal anti-inflammatory drugs.
References
- DailyMed - REGLAN- metoclopramide hydrochloride tablet. Nih.gov. Published 2010. Updated June 19, 2020. Accessed June 14, 2025. Link
- Rosenfeld MR, Dvorkin B, Klein PN, Makman MH. Differential affinities of molindone, metoclopramide and domperidone for classes of [3H]spiroperidol binding sites in rat striatum: evidence for pharmacologically distinct classes of receptors. Brain Res. 1982;235(1):205-211. PubMed
- Linnik MD, Butler BT, Gaddis RR, Ahmed NK. Analysis of serotonergic mechanisms underlying benzamide-induced gastroprokinesis. J Pharmacol Exp Ther. 1991;259(2):501-507. PubMed
- Freeman AJ, Cunningham KT, Tyers MB. Selectivity of 5-HT3 receptor antagonists and anti-emetic mechanisms of action. Anticancer Drugs. 1992;3(2):79-85. PubMed
- Ross-Lee LM, Eadie MJ, Hooper WD, Bochner F. Single-dose pharmacokinetics of metoclopramide. Eur J Clin Pharmacol. 1981;20(6):465-471. PubMed
- De Oliveira GS Jr, Castro-Alves LJ, Chang R, Yaghmour E, McCarthy RJ. Systemic metoclopramide to prevent postoperative nausea and vomiting: a meta-analysis without Fujii's studies. Br J Anaesth. 2012;109(5):688-697. PubMed
- Navari RM, Aapro M. Antiemetic Prophylaxis for Chemotherapy-Induced Nausea and Vomiting. N Engl J Med. 2016;374(14):1356-1367. PubMed
- Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures: An Updated Report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Anesthesiology. 2017;126(3):376-393. PubMed
- Solanki DR, Suresh M, Ethridge HC. The effects of intravenous cimetidine and metoclopramide on gastric volume and pH. Anesth Analg. 1984;63(6):599-602. PubMed
- Camilleri M, Kuo B, Nguyen L, et al. ACG Clinical Guideline: Gastroparesis. Am J Gastroenterol. 2022;117(8):1197-1220. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.