Copy link
Lung Point-of-Care Ultrasound
Last updated: 01/17/2023
Key Points
- Point-of-care ultrasound (POCUS) is a highly specific tool that can be used in the perioperative setting for real-time imaging and diagnosis of various pathologies (diagnostic POCUS) and guidance of certain percutaneous procedures (procedural POCUS).
- Diagnostic POCUS of the lung can be used to identify lung pathologies such as pneumothorax, interstitial syndrome, lung consolidation, and pleural effusions. These conditions can be diagnosed using POCUS with comparable or better accuracy than by radiographic imaging.1
- Due to the low cost and often pocket-sized convenience, POCUS is becoming a powerful tool for the early detection and management of various pathologies. It is critical for anesthesiologists to become well versed in using POCUS at the bedside.
Introduction
- Diagnostic POCUS is a powerful tool for evaluating various organ systems in real-time. Specifically, POCUS has become a mainstay in the evaluation and diagnosis of several lung pathologies.
- POCUS has widespread utility in numerous clinical areas, including the perioperative setting, emergency room, and intensive care unit (ICU).
- POCUS of the lung can be an invaluable tool both intraoperatively and postoperatively for the diagnosis and management of several conditions such as pneumothorax, pneumonia, and pulmonary edema with a high degree of sensitivity and specificity.1,2
Normal Lung Anatomy on Ultrasound
- Depending on the transducer used, lung POCUS can identify pathology at different depths:
- High-frequency transducers (vascular probes) are ideal for visualizing superficial pathology such as pneumothorax.
- Low-frequency transducers (curvilinear and transthoracic probes) can detect deeper pathology such as interstitial/alveolar edema, consolidation, and pleural effusion.
- When the transducer is oriented perpendicular to the rib spaces, the following structures are visualized in the normal lung (from superficial to deep): intercostal muscle, ribs, pleural line (hyperechoic thin line), and lung parenchyma.
Lung Sliding
- Lung sliding is a normal sign observed with 2-dimensional (2D) ultrasound when both
- the parietal and visceral pleura are directly opposed to one another, with no intervening air between them;
- and ventilation is occurring at the observed interspace.
- In this setting, lung ultrasound reveals a horizontal sliding phenomenon at the pleural line synchronous with the patient’s respiratory rate. This sliding at the pleural line represents the movement of the visceral pleura against the permanently static parietal pleura. Absence of lung sliding is abnormal and can be due to various causes, including the absence of ventilation at the examined location, pneumothorax, lung adhesions, or emphysematous blebs.
Lung Pulse
- Lung pulse is a normal finding wherein cardiac oscillations are seen at the pleural line as a small amplitude vertical oscillation synchronous with the patient’s heart rate. Lung pulse is seen when the visceral and parietal pleura are directly opposed to one another with no air (i.e., no pneumothorax) intervening between them.
A-Line
- A-line is a nonanatomic artifact seen in multiple conditions, including normal lung, obstructive lung disease, and pneumothorax. A-lines are reflections of the pleural line and appear as horizontal lines deep in the image at an integer multiple of the distance between the pleural line and skin (Figure 1).
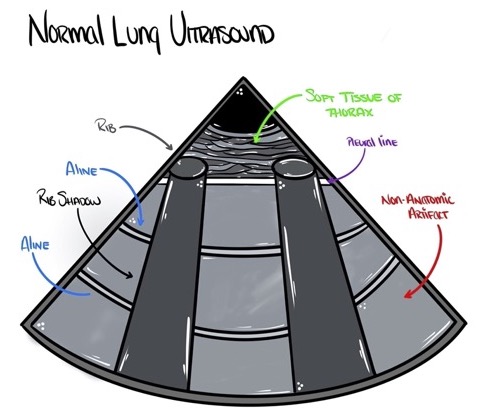
Figure 1. Anatomic correlates of normal lung ultrasound. Source: Author’s illustration. Used with permissions from David Convissar MD (www.countbackwardsfrom10.com)
Motion Mode (M-mode)
- M-mode is a form of greyscale ultrasound where the provider selects a single vertical line of tissue within the 2D image to interrogate. M-mode generates a rectangular box containing a live view of the line of tissue, with the y-axis of the box representing depth in the body and the x-axis representing time.
- M-mode provides spatial resolution limited to one dimension but an extremely high temporal resolution that can be helpful when evaluating fast-moving structures in the heart. The lung, however, has no fast-moving structures, so the utility of M-mode is unclear, and in fact, the only recommendations on lung ultrasound published to date omit any comment requiring the use of M-mode.1
- Nevertheless, M-mode has historically been emphasized in static journal articles on lung ultrasound, possibly because 2D representation of lung sliding is not possible using a printed still image but is possible using M-mode.
- The M-mode equivalent of lung sliding is the “seashore sign” (Figure 2), which resembles the ocean and sand on a seashore.
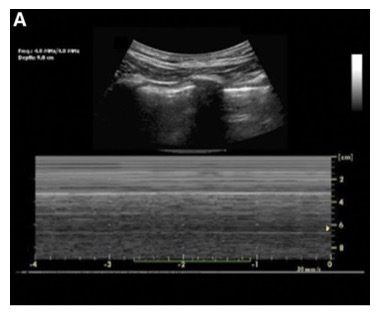
Figure 2. Normal lung ultrasound and concurrent M-mode showing the seashore sign.3
Standard Approach to the Lung POCUS Examination
- The minimum number of lung ultrasound views needed to perform a complete exam varies considerably depending on the clinical context.
- Current international lung ultrasound recommendations support a standardized eight-region sonographic lung exam while acknowledging that a rapid anterior two-region scan may be sufficient in the critically ill patient to rule out pulmonary edema. These 2-to-8 region exams are suggested in the acute setting due to their rapid nature and high sensitivity for interstitial syndromes (fluid in the lung), as compared to the more comprehensive 28-field scan performed in more stable settings. For the 8-region exam, scanning can be broken up into 2 upper and 2 lower regions, separated by the parasternal line, anterior axillary line, and posterior axillary line vertically, and an axial line just below the nipple.
- Alternatively, some authors have suggested a three-region exam per hemithorax in the perioperative and ICU setting (Figure 3).4
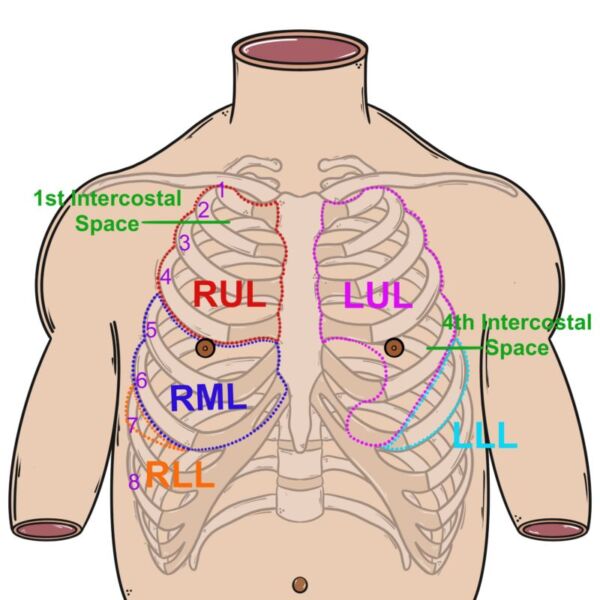
Figure 3. Regions to scan for the 6-region lung ultrasound. Source: Author’s illustration. Used with permissions from David Convissar MD (www.countbackwardsfrom10.com)
Left hemothorax:
(L1) mid-clavicular line, 2nd-3rd intercostal space (ICS),
(L2) anterior axillary line, 4th-6th ICS,
(L3) mid-to-posterior axillary line, 5th-7th ICS.
Right hemothorax:
(R1) mid-clavicular line, 2nd-3rd ICS
(R2) anterior axillary line, 4th-6th ICS
(R3) mid-to-posterior axillary line, 5th-7th ICS.
Pneumothorax on Lung Ultrasound
- Diagnostic POCUS is a powerful tool for the early detection of pneumothorax and has been demonstrated to be more specific and sensitive than a supine chest x-ray.
- There are four sonographic findings associated with a pneumothorax: (i) absence of lung sliding; (ii) absence of lung pulse; (iii) absence of B-lines; and (iv) possible presence of a lung point.
- Since a pneumothorax is a collection of air between the visceral and parietal pleura and reflects more than 99% of the ultrasound energy that reaches the first layer of air, a pneumothorax prevents visualization of anything deep to the parietal pleura. So, a pneumothorax prevents visualization of the movement of the visceral pleura during the respiratory cycle (lung sliding), cardiac pulsations that have percolated to the visceral pleura (lung pulse), and signs of pathology in the lung parenchyma such as B-lines (see interstitial syndrome below).
- Lung point is an abnormal finding sometimes seen at the edges of a pneumothorax. Lung point is thought to be pathognomonic for pneumothorax and describes an interspace where lung sliding is seen entering and then completely retreating from an otherwise completely static pleural line. In a lung point, the pleural line is completely static in between breaths with no lung sliding, no lung pulse, and no B-lines. On the ultrasound image, the static pleural line identifies the location of the pneumothorax, and the lung sliding identifies the normal aerated lung transiently displacing the pneumothorax in the interspace during inspiration (Figure 4). Of note, a lung point is only seen when the lung parenchyma can displace the pneumothorax during inspiration; therefore, lung point may not be seen when a pneumothorax reaches severe tension physiology.
- In suspected unilateral pneumothorax, comparison with the contralateral lung may help providers more confidently identify the ultrasound signs.
- Summary of ultrasound findings in a pneumothorax: (i) absence of lung sliding, (ii) absence of lung pulse, (iii) absence of B-lines, and (iv) possible presence of a lung point.
- Optimal scan site: start with the least gravitationally dependent area, and move laterally (e.g., L1/R1, then L2/R2 in Figure 3).
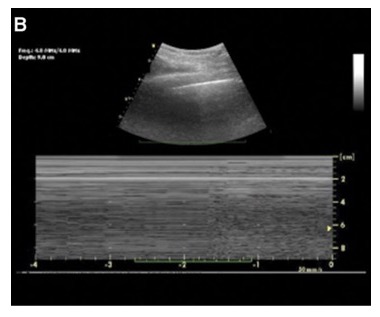
Figure 4. Lung ultrasound with lung point in pneumothorax and M-mode showing a barcode sign3
Interstitial Syndrome on Lung Ultrasound
- Interstitial syndrome is a sonographic term to describe the presence of fluid or fibrosis within the interstitium of the lung tissue.
- Interstitial syndromes can be focal (unilateral) or diffuse (bilateral).
Examples of focal (unilateral) interstitial syndrome include - early pneumonia, early atelectasis, pneumonitis, and pulmonary contusion.
- Examples of diffuse (bilateral) interstitial syndrome include
- hydrostatic pulmonary edema (e.g., cardiogenic pulmonary edema, negative-pressure pulmonary edema, transfusion-associated circulatory overload);
- nonhydrostatic pulmonary edema (e.g., acute respiratory distress syndrome, transfusion-associated circulatory overload);
- pulmonary fibrosis;
- bilateral pneumonia (including COVID-19).
- The hallmark finding on lung ultrasound in interstitial syndrome is the presence of B-lines. B-lines are vertical ring-down artifacts emanating from the pleural line and propagating down to the deepest portion of the ultrasound screen (Figures 5 and 6). B-lines efface A-lines where the two intersect. B-lines are correlated with lung density: the higher the number of B-lines in an interspace, the more that the normally thin interstitium of the lung has been filled in with some sort of density such as fluid or fibrosis.
- B-lines are considered pathologic when either of the following is true in each interspace
- 3 or more B-lines are present or
- a single confluent B-line occupies most of the interspace.
- Summary of ultrasound findings in interstitial syndromes: B lines are vertical ring-down artifacts emanating down from the pleural line and indicate increased lung density.
- Optimal scan site: start with the least gravitationally dependent area, and move laterally (e.g., L1/R1, then L2/R2 in Figure 3).
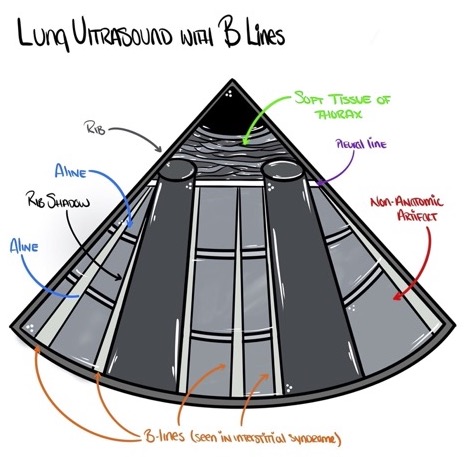
Figure 5. Lung ultrasound of interstitial syndromes showing multiple B-lines. Source: Author’s illustration. Used with permissions from David Convissar MD (www.countbackwardsfrom10.com)
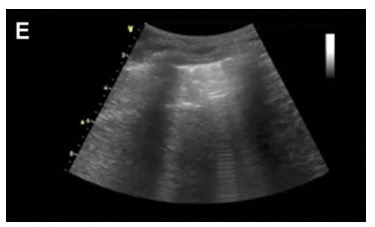
Figure 6. Lung ultrasound showing B-lines emanating from the pleural line.
Lung Consolidation on Ultrasound
- Lung consolidations are characterized on ultrasound by the finding of sonographic hepatization: the appearance within the lung parenchyma of liver-like tissue instead of the normal nonanatomic artifacts created by sonographically normal lung (Figures 7 and 8).
- Consolidations may be the result of several pathologies, including infiltrative processes (e.g., pneumonia or malignancy), atelectasis, and pulmonary embolism.
- Dynamic air bronchogram (DAE) is an additional finding that increases the probability of pneumonia over atelectasis. DAE are small, hyperechoic (bright) circles within the lung parenchyma that move with respiration. The movement of these circles implies the lung parenchyma in the area has ongoing airflow. The presence of airflow is more likely in infiltrative conditions such as pneumonia than in complete airway collapse, such as atelectasis.
- Of note, some lung consolidations do not reach the pleura and will not be visible on lung ultrasound.
- Optimal scan site: mid-to-posterior axillary line just cranial to the diaphragm (e.g., L3/R3 in Figure 3).
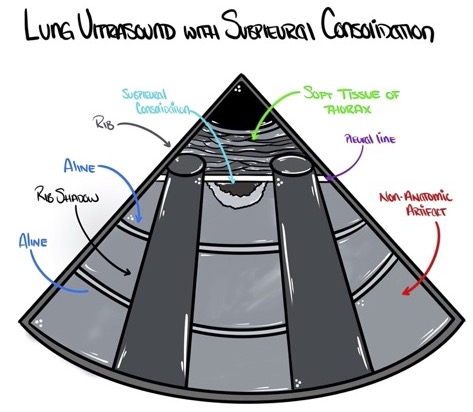
Figure 7. Subpleural lung consolidations appear as an echo-poor region under the pleural line. Source: Author’s illustration. Used with permissions from David Convissar MD (www.countbackwardsfrom10.com)
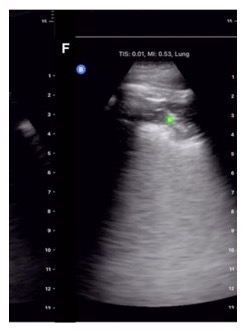
Figure 8. Lung ultrasound showing subpleural consolidation as indicated by the green dot.
Pleural Effusion on Lung Ultrasound
- Pleural effusions or collections of fluid within the pleural space are usually found within the dependent parts of the lung and can be well visualized using bedside ultrasound. By scanning the dependent regions of the lung depending on the patient’s position, pockets of fluid that are all black (Figures 9 and 10) may be seen. Internal echoes within the fluid may suggest a hemorrhagic or exudative effusion.1
- Spine sign is an abnormal finding seen on lung ultrasound where, in a posterolateral view of the lung (anterior-to-posterior axillary line, 5th-7th ICS), one can visualize the supradiaphragmatic spine. The supradiaphragmatic spine is only visible in this view if a continuous column of fluid and/or consolidated lung is present between the transducer and the supradiaphragmatic spine. Therefore, an ipsilateral spine sign is nearly always seen in the setting of a pleural effusion.
- Sinusoid sign is a visualization of the lung parenchyma floating in pleural fluid. Sinusoid sign is commonly seen in pleural effusions but can be missing if the volume of effusion displaces all lung parenchyma from the view.1
- Summary of ultrasound findings in pleural effusion: anechoic space between parietal and visceral pleura, spine sign, and possible sinusoid sign.
- Optimal scan site: mid-to-posterior axillary line just cranial to the diaphragm (e.g., L3/R3 in Figure 3).
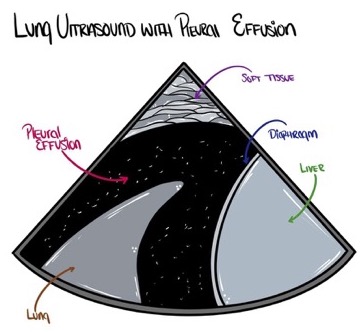
Figure 9. Pleural effusions often appear as a dark (anechoic or hypoechoic) space between the parietal and visceral pleura. Source: Author’s illustration. Used with permissions from David Convissar MD (www.countbackwardsfrom10.com)
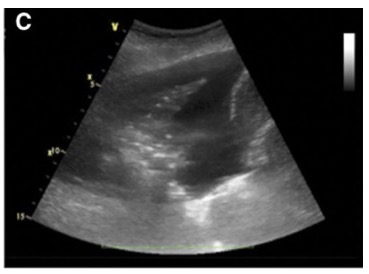
Figure 10. Lung ultrasound showing a pleural effusion.
- Diagnostic POCUS for the evaluation of lung pathology is becoming more mainstream in the perioperative setting, emergency rooms, and ICUs. POCUS is reliable at diagnosing or ruling out various pathologies, which can result in prompt management of critical patients.
- Therefore, anesthesiologists must become familiar with POCUS for lung evaluation.
References
- Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012; 38(4):577-91. PubMed
- Heldeweg MLA, Vermue L, Kant M, et al. The impact of lung ultrasound on clinical decision making across departments: a systematic review. Ultrasound J. 2022;14(1):5. PubMed
- Ford JW, Heiberg J, Brennan AP, et al. A pilot assessment of 3 point-of-care strategies for diagnosis of perioperative lung pathology. Anesth Analg. 2017;124(3):734-42. PubMed
- Fox WC, Krishnamoorthy V, Hashmi N, et al. Pneumonia: Hiding in plain (film) sight. J Cardiothorac Vasc Anesth. 2020;34(11):3154-57. PubMed
- Convissar DL, Gibson LE, Berra L, et al. Application of lung ultrasound during the COVID-19 pandemic: A narrative review. Anesth Analg. 2020;131(2):345-50. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.