Copy link
Hypoxia During One-Lung Ventilation
Last updated: 07/13/2023
Key Points
- One-lung ventilation (OLV) is used to facilitate surgical exposure in the thoracic surgical patient using a double-lumen endotracheal tube (DLT) or a bronchial blocker. During OLV, an obligatory shunt may produce hypoxemia secondary to the collapse of the nondependent lung and increased atelectatic areas in the dependent lung.
- This hypoxemic event will activate hypoxic pulmonary vasoconstriction (HPV), which leads to the contraction of vascular smooth muscle in the pulmonary circulation in response to a low regional partial pressure of alveolar oxygen, thus decreasing the shunt by redirecting pulmonary blood flow to the well-oxygenated and dependent lung.1
- Hypoxia during OLV is defined as a decrease in oxygen saturation (SaPO2) measured by pulse oximetry of less than 85% to 90% range value; usually episodes last a few minutes during OLV. Hypoxia during OLV can also be defined as an arterial oxygen tension (PaO2) of less than 60 mmHg when the patient is being ventilated at an inspired oxygen fraction (FiO2) of 1.0.
- Incidence of hypoxemia during OLV is currently estimated at less than 4% in part because of the use of flexible fiberoptic bronchoscopy to achieve an optimal position of the lung isolation devices.2 It is also attributable to the introduction of newer volatile anesthetics that cause less inhibition of HPV in dose-dependent manner and less venous admixture during OLV.3
- Individuals with coexisting cardiovascular, cerebrovascular, or pulmonary disease are undoubtedly at great risk for circulatory compromise caused by hypoxemia. Oxygen delivery to the myocardium may already be marginal in patients with severe coronary artery disease. Decrease in oxygen delivery can result in depressed myocardial function, and this can be seen during hypoxia and OLV.
Pathophysiology of Hypoxia During One-Lung Ventilation
- When OLV is initiated, the nondependent lung becomes atelectatic. Alveolar oxygen level decreases, and hypoxemia develops. During OLV, a shunt-like effect may arise from continued perfusion of the nonventilated lung and inadequate expansion of the ventilated or dependent lung while the patient is receiving a high inspired O2 fraction (FiO2 = 1.0) or from hemodynamic changes related to the anesthetic management and position during thoracic surgery.
- Hypoxemia during OLV is caused by venous admixture through shunts and areas of low V/Q gas exchanging units. Based on animal studies, the maximal HPV response during OLV decreases blood flow to the nondependent lung by 50%. During OLV, the estimated venous admixture is usually 20−25% of total cardiac output. Therefore, patients who are well oxygenated during two-lung ventilation will have a PaO2 of approximately 350−400 mmHg and, when converted to OLV, will have a PaO2 of approximately 150−200 mmHg while receiving FiO2 of 1.0.
HPV
- With the patient in a lateral decubitus position when both lungs are being ventilated, the proportion of the pulmonary blood flow is distributed as follows: The dependent (nonoperative) lung receives approximately 60% of the pulmonary blood flow (more perfusion), whereas the nondependent lung (operative lung) receives 40% of the total pulmonary blood flow. When OLV is instituted, the nondependent lung becomes atelectatic. Because the alveolar O2 decreases, there is a response to HPV in which increased pulmonary vascular resistance diverts blood flow towards the dependent lung.
- HPV is a biphasic reaction with an early response that usually starts within seconds and reaches a peak between 20−30 minutes during OLV, followed by a delayed response during the next 2 hours when the maximal vasoconstrictor response is achieved. Throughout this time, the PaO2 will gradually increase. However, during repeated episodes of OLV and re-expansion of the nondependent lung, the HPV reflex appears to be augmented. During these subsequent periods of OLV, oxygenation is thought to improve because the blood flow in the reinflated lung has not returned to baseline before the subsequent hypoxic challenge.4
- Figure 1 displays the physiology of OLV. HPV is an autoregulatory reflex mechanism that reduces pulmonary blood flow through the nondependent lung by 40−50% and diverts part of the blood flow to the dependent lung. Factors that will modify the HPV response during OLV include hypotension, hypocapnia, hypothermia, the presence and severity of chronic obstructive pulmonary disease (COPD), and to a minimal degree, the use of vasodilators, vasoconstrictors, and anesthetic agents.
- HPV is inhibited by several anesthetic agents.4
- All volatile anesthetics (halothane, sevoflurane, isoflurane, and desflurane) inhibit HPV in a dose-dependent fashion.
- Inhaled nitric oxide (NO) inhibits HPV secondary to its pulmonary vasodilatory effects.
- Nitroprusside and nitroglycerin are NO donors and inhibit HPV.
- Phosphodiesterase inhibitors such as sildenafil impede the breakdown of cyclic GMP, resulting in more NO and vasodilation.
- Calcium channel blockers inhibit HPV.
- Angiotensin-converting enzyme inhibitors also inhibit HPV.
- Prostacyclin is a potent pulmonary vasodilator.
- Of note, propofol has not been shown to inhibit HPV. The effects of nitric oxide on HPV are not clear.4
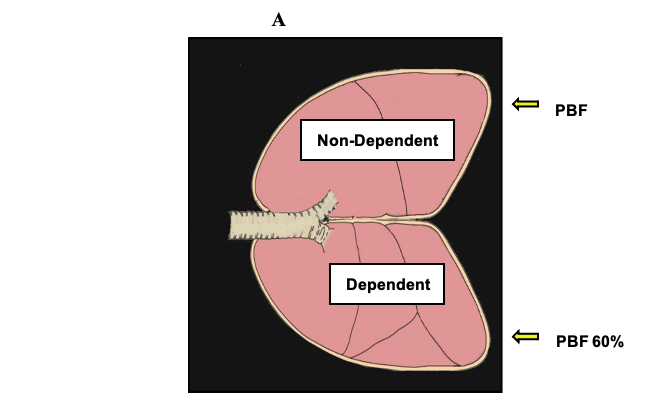
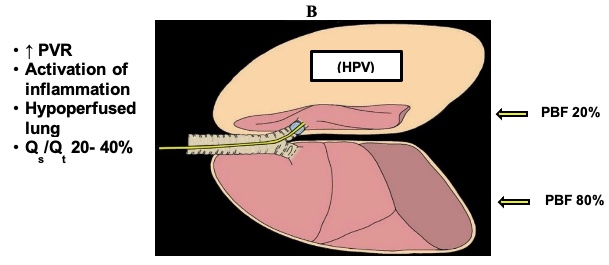
Figure 1. Physiology of OLV. PVR = pulmonary vascular resistance; PBF = pulmonary blood flow; Qs/Qt = shunt fraction. With permission from Campos JH, et al.1
Predictors of Hypoxemia
Factors that predict which patients are most likely to develop hypoxemia during OLV include:
- Side of the surgery: right-sided surgery (right lung collapse) and left-sided ventilation increases the risk of hypoxia because the right lung is approximately 10% larger than the left lung.
- Pulmonary function test is the percentage of forced expiratory volume in one second [FEV1]. An inverse correlation exists between FEV1 and PaO2.
- Low intraoperative PaO2, decreased values of PaO2 intraoperatively during two-lung ventilation in a lateral position while the patient is receiving 100% oxygen.
- Perfusing of the lungs: lung perfusion studies have demonstrated that the nonventilated and collapsed lung is more impaired in patients presenting for pneumonectomy than in patients who present with solitary lung nodes undergoing video-assisted thoracoscopic surgery.
- Body mass index (BMI): patients with a BMI greater than 30kg/m2 who require OLV develop more episodes of intraoperative and postoperative hypoxemia when compared with nonobese patients.
- Previous lobectomy on the contralateral side increases the risk of hypoxia because 25% of the lung function has been affected by previous lobectomy.
- Position of the patient during surgery: during OLV in a lateral decubitus position, gravity augments the redistribution of perfusion to the ventilated or dependent lung, improving and maintaining ventilation-perfusion (V/Q) matching.
- Table 1 lists the factors that increase the risk of hypoxemia during OLV.
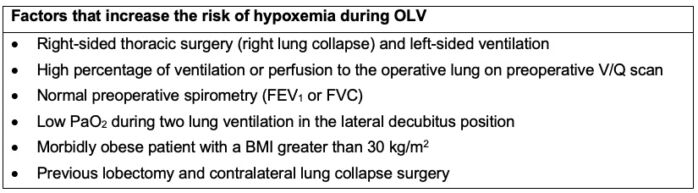
Table 1. Factors that increase the risk of hypoxemia during OLV. BMI = body mass index; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; OLV = one-lung ventilation; PaO2 = arterial oxygen tension. With permission Campos JH, et al.1
Management
- If hypoxia occurs during OLV, any nonurgent surgical procedure must be stopped, FiO2 should be increased to 1.0, and two-lung ventilation restored.
- After hypoxia is treated, a fiberoptic bronchoscopic exam should be performed to ensure that the DLT or bronchial blocker is in optimal position.
- Alveolar recruitment maneuvers should be used to improve arterial oxygenation. Lung recruitment is a ventilator maneuver aimed to reverse atelectasis by means of a brief, controlled increase in the airway pressures with expansion of the lungs. This maneuver will improve arterial oxygenation, decrease pulmonary shunt and dead space with adequate levels of positive end-expiratory pressure (PEEP).
- Application of continuous positive airway pressure (CPAP) to the nondependent lung has been suggested in the deflation phase of tidal volume (VT) breath. CPAP is thought to improve oxygenation by a passive mechanism (uptake of oxygen by the alveoli with continuous oxygen administration).
- Apneic oxygen insufflation with application of 3L of O2 via a suction catheter to the nonventilated side has improved oxygenation during OLV.5
- Other recommended maneuvers to manage hypoxia during OLV are listed in Table 2.
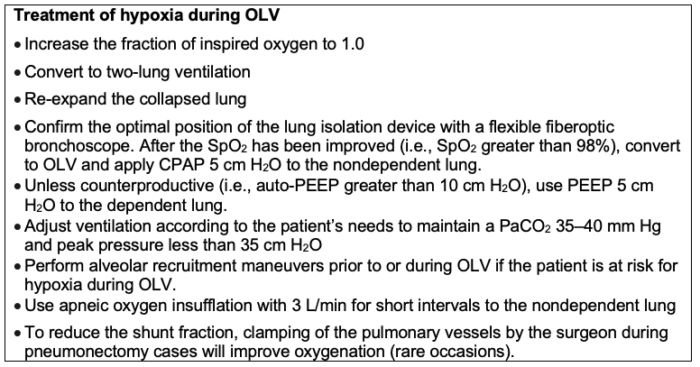
Table 2. Treatment of hypoxia during OLV. CPAP = continuous positive airway pressure; FiO2 = inspired oxygen fraction; OLV = one-lung ventilation; PaCO2 = arterial carbon dioxide tension; PEEP = positive end-expiratory pressure; SpO2 = arterial oxygen saturation.
References
- Campos JH, Feider A. Hypoxia during one-lung ventilation−A review and update. J Cardiothorac Vasc Anesth. 2018;32: 2330-8. PubMed
- Campos J. Fiberoptic bronchoscopy for positioning double-lumen tubes and bronchial blockers. In: Slinger P (ed). Principles and Practice of Anesthesia for Thoracic Surgery. 2nd edition. Switzerland. Springer. 2019: 311-22.
- Slinger PD, Campos J. Anesthesia for thoracic surgery. In: Gropper M, Cohen NH, Miller RD, et al. Miller's Anesthesia. Philadelphia, PA. 9th edition. Elsevier. 2019. 1648-1716.
- Lumb AB, Slinger P. Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology. 2015; 122:932-46. PubMed
- Jung DM, Ahn HJ, Jung SH, et al. Apneic oxygen insufflation decreases the incidence of hypoxemia during one-lung ventilation in open and thoracoscopic pulmonary lobectomy: A randomized controlled trial. J Thorac Cardiovasc Surg. 2017; 154:360-6. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.