Copy link
High-Altitude Illnesses
Last updated: 04/29/2025
Key Points
- The risk and severity of acute high-altitude illnesses are determined by the rate of ascent, individual susceptibility, and degree of acclimatization.
- Early recognition and prompt treatment with rest, oxygen, descent, and medication are critical in preventing progression to life-threatening illness.
- Chronic mountain sickness (CMS) is a distinct chronic condition resulting from long-term exposure to high-altitude hypoxia.
Introduction
- High-altitude illnesses arise from decreased arterial oxygen saturation and partial pressure of oxygen above 2500 meters, leading to hypobaric hypoxia and reduced oxygen delivery to tissues.
- Acute high-altitude illnesses include acute mountain sickness (AMS), high-altitude cerebral edema (HACE), and high-altitude pulmonary edema (HAPE).
- AMS and HACE are believed to exist along a clinical spectrum, with AMS commonly preceding the more severe form of HACE.1,2
- CMS represents a long-term maladaptation to high-altitude hypoxia, and its prevalence varies significantly depending on genetic and geographic factors.
Epidemiology and Risk Factors
- AMS affects approximately 25% of individuals ascending to 2500–3000 meters and up to 50% of those who ascend above 4000 meters.1,2
- Risk factors include rapid ascent, poor acclimatization, physical exertion at altitude, and individual susceptibility. Of note, pre-ascent physical conditioning does not decrease risk.1,2
- HACE, a severe progression of AMS, occurs in approximately 1% to 2% of individuals ascending to 4000 meters and is rare below 2500 meters.3
- Less than 1% of cases of AMS progress to HACE.3
- HACE shares the same risk factors as AMS.1,2
- HAPE has an incidence of 0.01–0.1% at 2500 meters, increasing to 2–6% when ascending to 4000 meters.3
- Risk increases with rapid ascent, male sex, cold exposure, cardiopulmonary disease, and reentry to high altitude after time at lower elevations.1
- CMS affects long-term residents above 3000 meters, with prevalence ranging from 1.2% in Tibetans to 33.7% in Andean populations, suggesting a genetic influence on disease development.1
Pathophysiology
- AMS is thought to result from central nervous system dysfunction secondary to hypoxia.1,2
- Central hypoxia leads to cerebral vasodilation and mild brain edema. Additionally, blood-brain barrier permeability is increased in AMS, which lowers oncotic pressure through protein leakage, contributing to cerebral edema.1,2
- Individuals with lower cerebrospinal fluid-to-brain ratios may be more susceptible to developing AMS.2
- HACE represents a severe extension of AMS, characterized by severe, vasogenic cerebral edema from continued blood-brain barrier breakdown.1,2
- Microhemorrhages, particularly in the corpus callosum, are a distinguishing feature of HACE from AMS and may indicate venous obstruction as a key pathological process of disease progression.1
- HAPE is believed to result from exaggerated hypoxic pulmonary vasoconstriction leading to elevated pulmonary artery pressure and noncardiogenic pulmonary edema.1,2
- Uneven pulmonary vasoconstriction and reduced nitric oxide availability contribute to elevated pulmonary vascular pressures and the subsequent development of edema.2
- Pulmonary artery pressures can exceed 60 mm Hg in susceptible individuals.1
- CMS develops from chronic hypoxia at altitude, which triggers excess erythropoietin production that results in polycythemia and increased blood viscosity.1
- Genetic predispositions such as SENP1 gene variants and poor ventilatory acclimatization may contribute to disease development.1
Clinical Features and Diagnosis
- AMS typically presents 4–12 hours after ascent to high altitude and presents with headache, fatigue, nausea, dizziness, insomnia, and anorexia.1,2
- Symptoms peak after the first night and resolve with rest in 1–3 days.3
- Headache is the hallmark symptom of AMS. The Lake Louise Score (LLS) is commonly used in research and may aid clinical assessment, with a score ≥3 with headache being diagnostic for AMS (Table 1).1-3
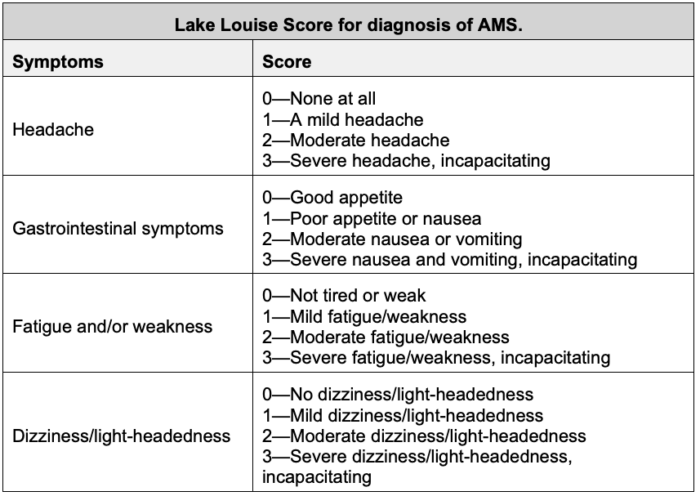
Table 1. Lake Louise Score. Adapted from Savioli G, Ceresa IF, Gori G, et al. Pathophysiology and therapy of high-altitude sickness: Practical approach in emergency and critical care. J Clin Med. 2022;11(14):3937.
- HACE presents 3-5 days after ascent and presents as worsening AMS with ataxia, confusion, irritability, drowsiness, and potentially coma.1,3
- Neurologic exam findings such as truncal ataxia, papilledema, cranial nerve palsies, or retinal hemorrhages may be present.2,3
- Magnetic resonance imaging may show T2/FLAIR hyperintensities of the corpus callosum.2
- HAPE presents 2–4 days after ascent, often without preceding AMS. Symptoms include dry cough and exertional dyspnea, which may progress to rest dyspnea, pink frothy sputum, and cyanosis.1,2
- Exam findings include tachypnea, tachycardia, fever, and inspiratory crackles.1-3
- Radiographic findings are not specific for HAPE; however, chest radiographs may reveal bilateral patchy infiltrates, and echocardiography may show evidence of right-ventricular strain.2
- CMS presents with fatigue, dyspnea, cyanosis, headache, and reduced exercise tolerance. Longstanding disease can progress to right heart failure and pulmonary hypertension.1
- Hemoglobin levels often exceed 21 g/dL in men and 19 g/dL in women.1
- A summary of clinical and diagnostic features is presented in Table 2.

Table 2. Summary of clinical findings in high-altitude illnesses.1-3 Abbreviations: MRI = magnetic resonance imaging, ICP = intracranial pressure, CXR = chest X-ray, ESR = erythrocyte sedimentation rate.
Prevention and Treatment
- Primary prevention of all acute high-altitude illnesses is centered around gradual ascent (300–600 meters per day above 3000 meters) with rest days every 2–3 days.1,2
- Sleeping at lower altitudes, adequate hydration, oxygen supplementation, and pre-acclimatization are effective nonpharmacological prevention strategies.1
- Adjunct pharmacologic prophylaxis may be considered for high-risk individuals.
- Acetazolamide is first-line pharmacologic prophylaxis for AMS and HACE. Dexamethasone is considered an alternative or adjunct for prevention.1,2
- Nifedipine is first-line pharmacologic prophylaxis for HAPE. Salmeterol and tadalafil are potential adjuncts.1,2
- Treatment of acute high-altitude illness begins with rest, immediate descent, and symptomatic therapy such as nonsteroidal anti-inflammatory drugs or antiemetics.1-3
- Supplemental oxygen or hyperbaric oxygen, if available, should be started if descent is not immediately possible.1,2
- A diagram for the management algorithm of acute high-altitude illnesses is shown in Figure 1.
- Pharmacologic treatment is appropriate for severe AMS, HACE, and HAPE, with specific therapies summarized in Table 3.4
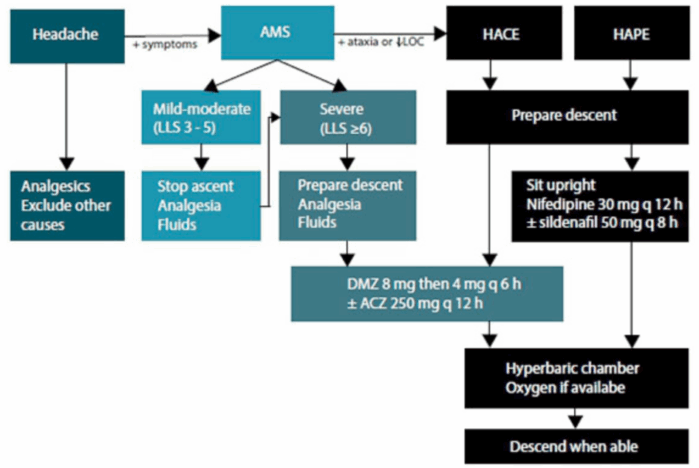
Figure 1. Flow diagram for the management of acute high-altitude illness. Source: Basnyat B, Hofmeyr R, Tölken G, De Decker R. Acute high-altitude illness. S Afr Med J. 2017;107(12):1047-1048. Published 2017 Nov 27. CC BY-NC 4.0. Link
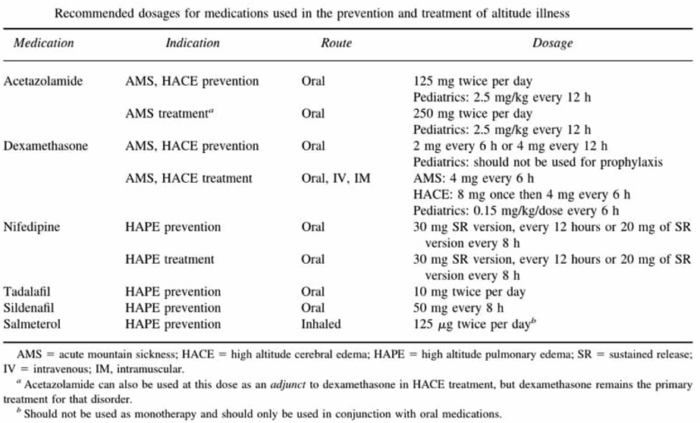
Table 3. Summary of pharmacologic prevention and treatment for high-altitude illnesses. Source: Nickson C. LITFL. CC BY-NC-SA 4.0. Link
References
- Cumpstey AF, Alexander J, Grocott M. Clinical Care in Extreme Environments: Physiology at high altitude and in space. In: Gropper MA, et al (eds). Miller’s Anesthesia. 9th ed. Elsevier; 2019: 2313-2336.
- Basnyat B, Murdoch DR. High-altitude illness. Lancet. 2003;361(9373):1967-74. PubMed
- Savioli G, Ceresa IF, Gori G, et al. Pathophysiology and therapy of high-altitude sickness: Practical approach in Emergency and Critical Care. J Clin Med. 2022;11(14):3937. PubMed
- Luks AM, McIntosh SE, Grissom CK, et al. Wilderness Medical Society consensus guidelines for the prevention and treatment of acute altitude illness [published correction appears in Wilderness Environ Med. 2010 Dec;21(4):386]. Wilderness Environ Med. 2010;21(2):146-155. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.