Copy link
Hepatic Drug Metabolism and Cytochrome P450
Last updated: 01/04/2023
Key Points
- The cytochrome P450 (CYP450) system describes a group of enzymes found predominantly in the liver that are responsible for the metabolism of most drugs in clinical use.
- CYP450 enzymes are typically involved in phase I oxidation, reduction, and hydrolysis reactions within the liver.
- Drug-drug interactions can result in the induction or inhibition of CYP450 enzymes which can produce undesirable clinical effects.
- Liver disease reduces CYP450 activity in complex and unpredictable ways, and thus, drugs metabolized in the liver should be used with caution in this context.
Introduction
- The CYP450 system refers to a collection of enzymes that play a major role in the metabolism of most drugs used in medicine. They also play a role in the synthesis and breakdown of endogenous substances such as steroid hormones.
- By nature, these enzymes catalyze reactions, most commonly within the liver, that either deactivate drugs or, in some cases, activate prodrugs.
- Knowledge of CYP450 metabolism is clinically applicable and crucial to the safe administration of medications, especially in scenarios in which drug-drug interactions may cause conflict or a patient’s CYP450 system is inherently deranged such as in liver disease.
CYP450 System
- The CYP450 system is composed of a large family of different enzymes which function as monooxygenases to oxidize substances in various metabolic pathways.
- The nomenclature of the CYP450 enzymes is delineated by gene sequences. They are assigned a family number (e.g., CYP1, CYP2), a subfamily letter (e.g., CYP1A, CYP2D), followed by a number for the individual protein (e.g., CYP1A1, CYP2D6).1
- While CYP450 enzymes are located throughout the body, they are highly concentrated within the smooth endoplasmic reticulum of hepatocytes within the liver.
- CYP450 enzymes are involved in the synthesis of numerous endogenous substances including steroids, cholesterol, prostacyclins, and thromboxane A2.
- CYP450 enzymes are perhaps most well known for their role in drug metabolism and are responsible for the metabolism of approximately 70-80% of all drugs in clinical use.2
- These enzymes most commonly catalyze the oxidative biotransformation of compounds which typically results in the deactivation of drugs but can also result in the activation of prodrugs (with medications such as codeine or clopidogrel).
CYP450 and Anesthetic Drugs
- The most important CYP for anesthetic drugs is CYP 3A4, which metabolizes fentanyl, alfentanil, sufentanil, midazolam, methadone, dexamethasone, lidocaine, and acetaminophen.
- Propofol is partially oxidized by CYP 3A4, but mostly by CYP 2B6.
- CYP 2D6 converts codeine, tramadol, oxycodone, and hydrocodone into their active metabolites.
- CYP 2E1 is primarily responsible for the oxidative metabolism of volatile anesthetics.
Hepatic Metabolism of Drugs
- Hepatic metabolism of drugs occurs in reactions categorized as phase I, phase II, and phase III reactions.
- Phase I reactions are primarily catalyzed by CYP450 enzymes that convert lipophilic drugs to hydrophilic molecules through oxidation, reduction, or hydrolysis. Non-CYP450 enzymes such as monoamine oxidase, alcohol dehydrogenase, and aldo-keto reductase are also involved in phase I reactions.3
- Phase II reactions are primarily catalyzed by uridine 5’-diphospho-glucuronosyltransfereases that conjugates the products of phase I pathway with hydrophilic moieties (e.g., glucuronic acid) to make them more water soluble.4
- Phase III reactions involve the excretion of compounds into bile by molecular transporters such as multidrug resistance protein, cystic fibrosis transmembrane conductance regulator, and multidrug-resistance-related protein.3
- The hepatic metabolism of drugs depends on several factors:3
- Age: expression and function of several phase I and II enzymes are reduced in neonates.
- Gender: the activities of some CYP450 enzymes are increased in women.
- Pregnancy: changes in drug metabolism during pregnancy are complex with increased activity of some CYP450 enzymes (such as CYP2D6 and CYP3A4) and decreased activity in others (such as CYP1A2).6
- Liver disease (see below)
- Genetic polymorphisms of metabolic enzymes results in wide variations in the pharmacokinetics of certain drugs like warfarin, codeine, tramadol, etc.
- Concomitant administration of drugs can serve as inducers or inhibitors of different enzymes (see below).
Drug-Drug Interactions
- Certain drug-drug interactions can result in the induction or inhibition of CYP450 enzymes.
- Induction of enzymes typically results from chronic exposure to drugs that are substrates of CYP450 enzymes resulting in increased enzyme production and thus significantly increased metabolism.4
- Enhanced metabolism can result in reduced drug efficacy for drugs ultimately deactivated by CYP450 enzymes or increased drug efficacy for prodrugs activated by CYP450 enzymes.
- Conversely, substances that cause inhibition of CYP450 enzymes will result in reduced metabolic activity and increased drug efficacy (or decreased efficacy of prodrugs).4
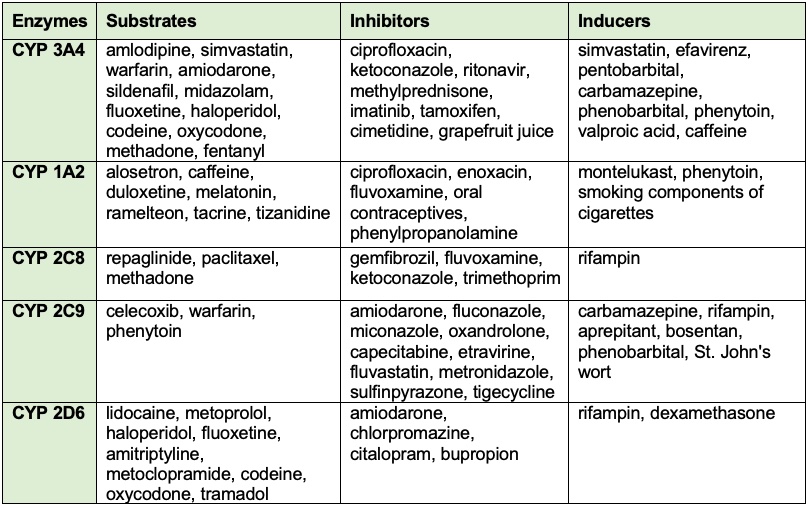
Table 1. Common drugs and substances that can cause induction or inhibition of CYP450 enzymes and their affected substrates.3,5
Drug Metabolism in Liver Disease
- Liver disease can lead to reduced CYP450 enzymatic activity.
- Unlike kidney injury where renal function can be approximated by creatinine clearance, the complex nature of hepatic blood flow and drug metabolism makes predictions of metabolic outcomes very difficult in acute or chronic liver disease.
- Drugs which are metabolized by the liver should be used with caution in the setting of liver disease as their clinical effects may be unpredictable.
References
- McDonnell AM, Dang CH. Basic review of the cytochrome P450 system. J Adv Pract Oncol. 2013;4(4):263-8. PubMed
- Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138(1): 103-41. PubMed
- Njoku DB, Chitilian HV, Kronish K. Hepatic Physiology, Pathophysiology, and Anesthetic Considerations. In: Gropper MA, ed. Miller’s Anesthesia. 9th ed. Elsevier, Inc.; 2020: Chapter 16. Pages 420-443.
- Forman SA, Ishizawa Y. Inhaled Anesthetic Uptake, Distribution, Metabolism and Toxicity. In: Gropper MA, ed. Miller’s Anesthesia. 9th ed. Elsevier, Inc.; 2020: Chapter 20. Pages 509-539.
- Gudin J. Opioid Therapies and Cytochrome P450 Interactions. J Pain Symptom Manage. 2012;44(6 Suppl): S4-S14. PubMed
- Isoherranen N, Thummel KE. Drug metabolism and transport during pregnancy: how does drug disposition change during pregnancy and what are the mechanisms that cause such changes? Drug Metab Dispos. 201341(2):256-62. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.