Copy link
Hemifacial Microsomia and Goldenhar Syndrome
Last updated: 01/12/2023
Key Points
- Hemifacial microsomia (HFM) and the related Goldenhar syndrome result from unilateral underdevelopment of structures originating from the first and second branchial arches, leading to a facial deformity of varying severity that often requires multiple corrective surgeries.
- These craniofacial anomalies can result in difficult airways that tend to worsen with age, and advanced airway management techniques may be required.
- Some patients have associated congenital malformations emanating from lower branchial arches and/or are extracraniofacial and can involve multiple organ systems.
Introduction
- HFM and Goldenhar syndrome are terms that are sometimes used interchangeably and are characterized by malformations of the orbit, mandible, ear, facial nerve, and soft tissue (OMENS) originating from the first and second branchial arches. Therefore, they are sometimes also referred to as first and second branchial arch syndromes.1
- HFM is a type of craniofacial microsomia characterized by varying degrees of unilateral hypoplasia of structures in a constellation that satisfies the minimum diagnostic criteria of:
- ipsilateral mandibular and ear (external/middle) defects; or
- asymmetrical mandible or ear (external/middle) defects in association with two or more indirectly associated anomalies or a positive familial history of HFM.1
- Goldenhar syndrome was first described in 1952 by Maurice Goldenhar. The syndrome description, in addition to features of HFM, included epibulbar dermoids, auricular appendices, preauricular fistulas, and vertebral anomalies. In 1963, Gorlin proposed the term “oculo-auriculo-vertebral dysplasia” (OAVS) instead, which points out the main features of this syndrome instead of the eponym.1
- Thus, OAVS is a variant of HFM that has added craniofacial or extracraniofacial (OMEN-plus) features such as vertebral bone anomalies or cardiac malformations.1-3
- The incidence of OAVS is estimated to be around 3.8 cases per 100,000 live births.1,2
- The etiology is likely genetic, caused by a sporadic mutation (not typically inherited), although nongenetic causes have also been hypothesized.1
- Heterogeneity of presentation with incomplete penetrance and variable expressivity makes genetic evaluation and diagnosis challenging.
Characteristics and Features
- Mal- and underdevelopment of the structures of the first and second branchial arches (Figure 1) is usually easy to recognize, especially when unilateral and severe. Anomalies of the external ear structures, such as periauricular tags, pits, or fistulas, should prompt clinicians to look for subtle unilateral or bilateral mandibular maldevelopment (Figure 2).
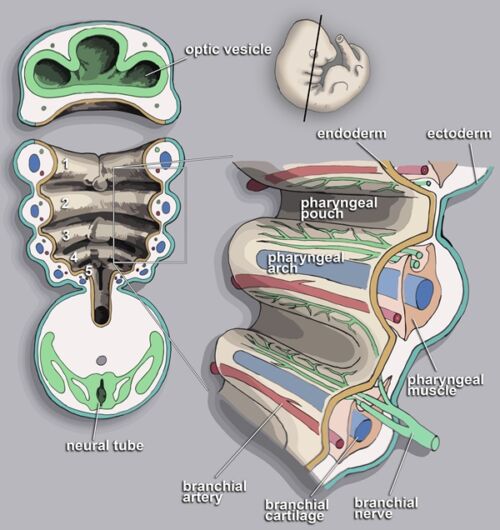
Figure 1. Embryonic origin of branchial arch structures. Adapted from Wikipedia: Loki Austenfell. CC-BY-SA-3.0.
- Ear deformity is a minimum diagnostic criterion and can range from the absence of external auditory canal to anotia, resulting in conductive and sensorineural hearing loss.
- Maxillofacial and mandibular hypoplasia are characteristic and can vary in severity. The temporomandibular joint may also be affected.
- Eye anomalies (epibulbar dermoids) can be present.
- Most cases are unilateral – hence the term HFM – leading to an asymmetry of the face, but bilateral involvement is possible.
- The maldevelopment is not limited to bony structures. Nerves (cranial nerves V and VII), soft tissues, and glands (parotid, thyroid) can also be impacted.
- Other features can include cleft lip and palate, and preauricular tags.
- An Expanded Goldenhar Complex has been described by various authors and is associated with some uncommon but clinically important associated anomalies:1,2
- cardiovascular malformations have been reported in 5%–58% of patients (tetralogy of Fallot, septal defects, and situs inversus)3;
- lung hypoplasia with altered respiratory mechanics;
- associated neuromuscular disease;
- laryngeal anomalies;
- skeletal anomalies, including vertebral (40% of patients), with the potential for cervical spine involvement;
- gastrointestinal anomalies; and
- renal anomalies.
- The airway abnormalities present in HFM/OAVS patients typically worsen with age.
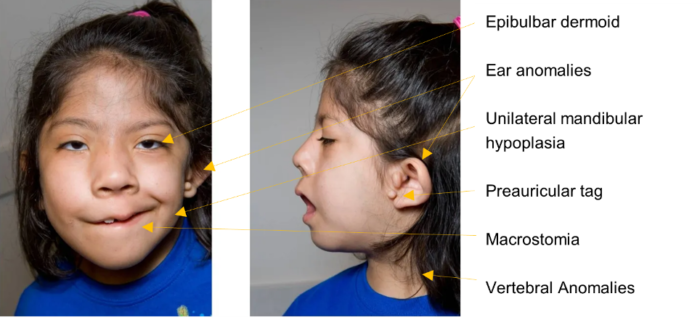
Figure 2. Frontal and lateral views of a patient with OAVS. Adapted from De Golovine S. Wu S, Hunter JV, et al. Goldenhar syndrome: a cause of secondary immunodeficiency? Allergy Asthma Clin Immunol. 2012; 8(1):10. CC BY 2.0
Surgical Management
- OAVS/HFM is/are complex disorder(s) warranting multidisciplinary care with multiple steps to surgical correction.
- The primary goal of any surgical intervention is the functional restoration of airway patency, occlusion and mastication, hearing, and speech. The secondary goal is the restoration of facial symmetry and aesthetic rehabilitation.
- Presurgical workup often includes diagnostic modalities requiring anesthesia, such as craniofacial imaging (magnetic resonance imaging or computed tomography), hearing test, drug-induced sleep endoscopy, microdirect laryngoscopy, bronchoscopy, etc.
- In cases of severely impaired airway patency, mandibular distraction osteogenesis (MDO) or tracheostomy might have to be performed in the neonatal period.
- Reconstructive surgery via a two-stage autologous rib cartilage graft technique is performed at age 7 or after and is typically preferred to the alternatives, which include observation or prosthetic replacement.
- Pedicled free flaps or fat grafting can be performed for the correction of soft tissue deformities.
- Various maxillofacial and dental procedures might be required throughout childhood to achieve satisfactory dental occlusion.
Anesthetic Considerations
- The main consideration is that mandibular hypoplasia, temporomandibular joint hypomobility, craniovertebral anomalies, separately or in combination, can result in challenging endotracheal intubation. Therefore, a difficult airway should be anticipated.
- The incidence of difficult intubation is 30-70%, increasing with complex defects, bilateral involvement, and older age.2,4
- Difficulty with mask ventilation is rare but possible.4
- In patients with vertebral involvement, cervical spine stability may be impacted, and appropriate imaging should be performed prior to elective airway management.3
- The presence of associated cardiac malformations may require special consideration in the anesthetic conduct that is specific to the type of anomaly to maintain hemodynamic stability.
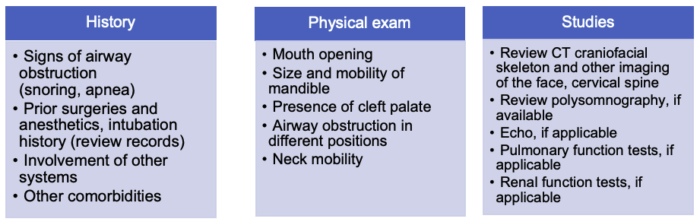
Table 1. Preoperative Evaluation
Preoperative Set-Up
- Difficult airway equipment such as oro- or nasopharyngeal airway, video/indirect laryngoscope, flexible bronchoscope, and supraglottic airways should be readily accessible in the operating room.
- Requesting the presence of an ENT surgeon and an additional anesthesiologist or qualified clinician should be considered during airway management.
Induction and Conduct of Anesthesia
- As most patients are developmentally normal (not delayed), the risk-benefit profile of premedication use would favor omitting it if severe obstructive sleep apnea is present.
- Attention should be paid to ensure that adequate preoxygenation is achieved prior to induction of general anesthesia, and supplemental oxygen should be administered during prolonged intubation attempts.
- Intravenous induction is preferred over inhalational induction, especially in older and larger patients due to the potential for difficult mask ventilation and/or difficult intubation. Spontaneously breathing inhalation induction may be considered in select infants and younger children who are more likely to maintain patency.
- Ketamine and dexmedetomidine may be useful adjuncts to any hypnotic agent, given their properties that help maintain airway patency and preserve the respiratory drive.
- Topicalization of the vocal cords with atomized lidocaine can improve intubation conditions in patients whose airway is managed without neuromuscular blocking agents.
- The pediatric difficult airway algorithm for intubation should be followed (Figure 3).
- The number of direct laryngoscopy attempts should be limited to no more than two.
- These patients benefit from a multimodal pain management strategy, including acetaminophen and nonsteroidal anti-inflammatory drugs (barring contraindication from extracraniofacial associated anomalies) to reduce the likelihood of opioid-induced postoperative airway obstruction. When required, opioids should be titrated to effect in small, incremental doses.
- Awake extubation is preferred, and airway patency should be monitored closely.
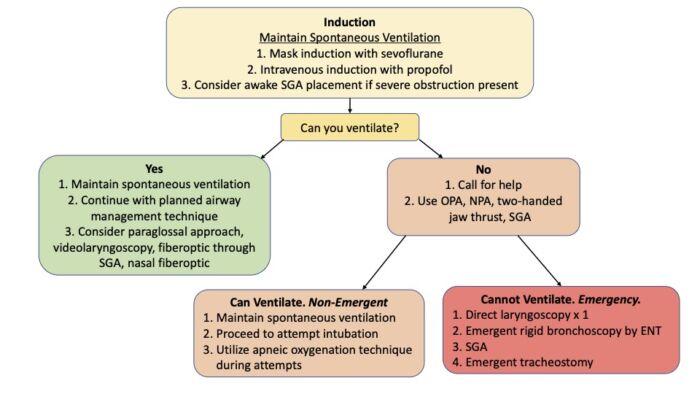
Figure 3. Algorithm for airway management in patients with HFM and Goldenhar syndrome. Abbreviations: OPA = oropharyngeal airway, NPA = nasopharyngeal airway, SGA = supraglottic airway. Adapted from Cladis F, et al. Pierre Robin sequence: a perioperative review. Anesth Analg. 2014;119(2):400-412.
Same algorithm applied in Snyder H, Hunyady A. Pierre Robin sequence. Link.
References
- Tingaud-Sequeira A, Trimouille A, Sagardoy T, et al. Oculo-auriculo-vertebral spectrum: new genes and literature review on a complex disease, J Med Genet 2022; 59:417-27. PubMed
- Stricker PA, Fiadjoe JE, Lerman J. Plastic and reconstructive surgery. In: Cote CJ, Lerman J, Anderson BJ. A Practice of Anesthesia for Infants and Children. 6th edition. Philadelphia, PA. Elsevier. 2019: 812-814.
- Choudhury M, Kapoor PM. Goldenhar syndrome: Cardiac anesthesiologist's perspective. Ann Card Anaesth. 2017;20(Supplement): S61-66 PubMed
- Xu J, Deng X, Yan F. Airway management in children with hemifacial microsomia: a retrospective study of 311 cases. BMC Anesthesiol. 2020;20(1):120. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.