Copy link
Hematologic Changes and Diseases in Pregnancy
Last updated: 10/07/2025
Key Points
- Anemia is the most common hematologic disorder in pregnancy.
- The physiological adaptations of pregnancy significantly alter hematologic parameters, which can complicate the interpretation of laboratory results and the management of pre-existing or newly diagnosed conditions.
- The patient’s bleeding history is a crucial factor to consider when determining the safety of neuraxial anesthesia.
Physiologic Hematologic Changes in Pregnancy
- Normal pregnancy is characterized by a “physiologic anemia” due to a disproportionate increase in plasma volume compared to red blood cell mass. This leads to a decrease in hemoglobin (Hb) concentration and hematocrit.
- Other notable changes include:
- Increased red blood cell mass: the total red cell mass increases by 20-30%.1
- Increased plasma volume: Increases by 40-50%.
- Leukocytosis: White blood cell counts increase, particularly neutrophils, reaching up to 12,000-15,000/µL in the third trimester and even higher during labor.
- Hypercoagulable state: Pregnancy is a prothrombotic state characterized by increased levels of most clotting factors (e.g., fibrinogen, factors VII, VIII, and X), decreased levels of natural anticoagulants (e.g., protein S), and impaired fibrinolysis. This is a protective mechanism against peripartum hemorrhage but increases the risk of venous thromboembolism (VTE).
- Thrombocytopenia: A mild, GT is common, with platelet counts typically remaining above 100,000/µL.1
Anemias of Pregnancy
- Anemia is the most common hematologic disorder in pregnancy, defined as a Hb concentration less than 11 g/dL in the first and third trimesters, and less than 10.5 g/dL in the second trimester.2
Iron Deficiency Anemia (IDA)
- Etiology: Accounts for 90% of anemias in pregnancy, primarily due to increased iron demands for fetal growth and maternal erythropoiesis.
- Diagnosis: Low ferritin, low serum iron, high total iron-binding capacity, microcytic hypochromic red blood cells.
- Management: Oral iron supplementation is the first-line treatment. Intravenous iron may be considered for severe anemia or intolerance to oral iron.3
- Anesthetic Implications: Severe IDA (Hb less than 7 g/dL) increases the risk of maternal cardiac decompensation, particularly with labor and delivery. Optimizing Hb levels prior to delivery is crucial. Consider transfusion if symptomatic or predelivery Hb is critically low.
Sickle Cell Disease
- Pathophysiology: Autosomal recessive disorder leading to abnormal hemoglobin S (HbS), causing red blood cell sickling under hypoxic, acidic, hypothermic, or dehydrated conditions.
- Pregnancy Complications: Increased risk of vaso-occlusive crises, acute chest syndrome, preeclampsia, preterm birth, fetal growth restriction, and stillbirth.5
- Anesthetic Management:
- Preoperative Optimization: Hydration, oxygenation, pain control, and transfusion to achieve HbS less than 30-40% if high-risk.
- Regional Anesthesia: Generally preferred for labor and delivery due to improved oxygenation and avoidance of systemic opioids. Careful attention to hydration and maintaining normothermia.
- General Anesthesia: Increased risk of sickling crisis. Meticulous attention to oxygenation, hydration, normocapnia, and normothermia. Avoidance of hypothermia, acidosis, and hypotension.
- Postpartum: Continue close monitoring for pain crises, VTE, and infection.
Thalassemia
- Pathophysiology: Inherited disorders of Hb synthesis (alpha or beta chains) that result in defective Hb, erythrocyte damage, and an imbalance in the globin chain ratios. Severity varies from asymptomatic carriers to transfusion-dependent forms.
- Pregnancy Complications: Depending on the severity of thalassemia and the type, patients have a higher incidence of spontaneous abortion, intrauterine fetal demise, and fetal growth restriction.
- Anesthetic Implications:
- Patients with transfusion-dependent thalassemia will require transfusion therapy through pregnancy. Even asymptomatic carriers may require transfusion during pregnancy.
- Manage chronic anemia and potential cardiac dysfunction (dilated cardiomyopathy from iron overload can often take place).
- Assess transfusion history and antibody status.5
Thrombocytopenia in Pregnancy
- Thrombocytopenia (platelet count less than 150,000/µL) is common in pregnancy, though often mild.4
GT
- The most frequent cause (75%), typically mild (more than100,000/µL), asymptomatic, and resolves postpartum.
- Regional anesthesia is generally safe if the platelet count is above 50,000-70,000/µL and a negative bleeding history, per the Society of Obstetric Anesthesiology and Perinatology consensus statement, though institutional guidelines may vary.
- The patient’s bleeding history is an extremely important factor to consider when determining if neuraxial anesthesia is safe.
- The quality of platelets is uncompromised.
Immune Thrombocytopenia
- An autoimmune disorder with antibody-mediated platelet destruction. Maternal bleeding risk is increased, but severe neonatal thrombocytopenia is rare. Treatment includes corticosteroids or IVIG.
- Regional anesthesia requires careful consideration of platelet count, typically aiming for a platelet count above 70,000/µL and a negative bleeding history.5
- Though platelet morphology may change, the quality of platelets is uncompromised.
HELLP Syndrome (Hemolysis, Elevated Liver Enzymes, Low Platelets)
- A severe form of preeclampsia. It is a life-threatening condition for both mother and fetus. Definitive treatment is delivery.
- Regional anesthesia is contraindicated if the platelet count is below 50,000/µL or rapidly decreasing. Though there are no guidelines for the frequency of obtaining platelet counts (studies have suggested that counts can change rapidly every 6-12 hours), clinical pictures can rapidly deteriorate, and platelet counts can rapidly fall.5
- A thorough airway exam and availability of difficult airway equipment at the time of delivery is encouraged.
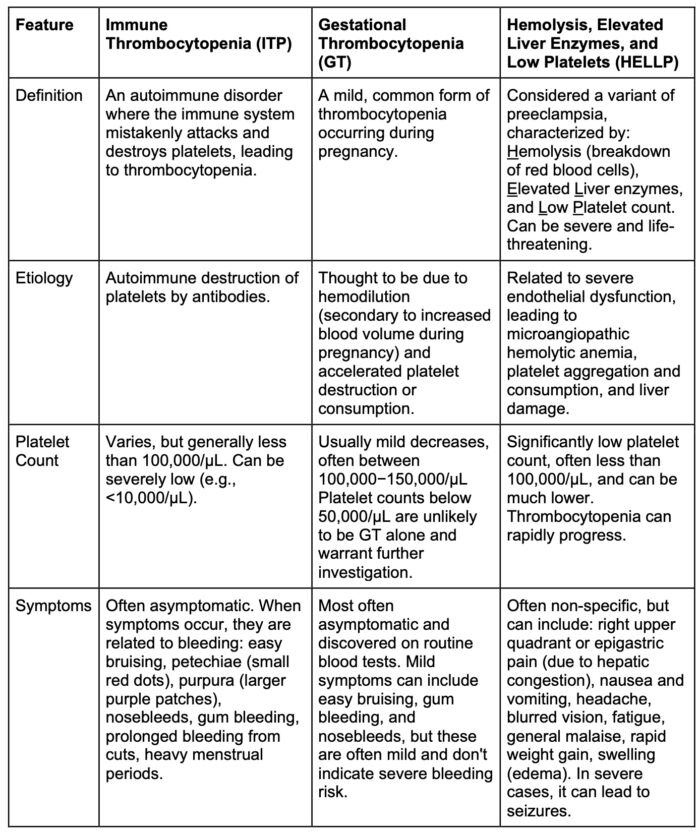
Table 1. ITP vs GT vs HELLP syndrome
Coagulopathies
Von Willebrand Disease (vWD)
- The most common inherited bleeding disorder, characterized by quantitative or qualitative defects in von Willebrand factor (vWF).5
- vWF levels typically increase during pregnancy, which can improve hemostasis, but postpartum hemorrhage remains a significant concern due to the rapid decline in vWF levels.
- Type 1 VWD is the most common, and its management often involves desmopressin (DDAVP) or factor VIII/vWF concentrates for severe cases or those unresponsive to DDAVP.5 Anesthetic planning includes assessing baseline factor levels and ensuring adequate levels peridelivery, especially for regional anesthesia.
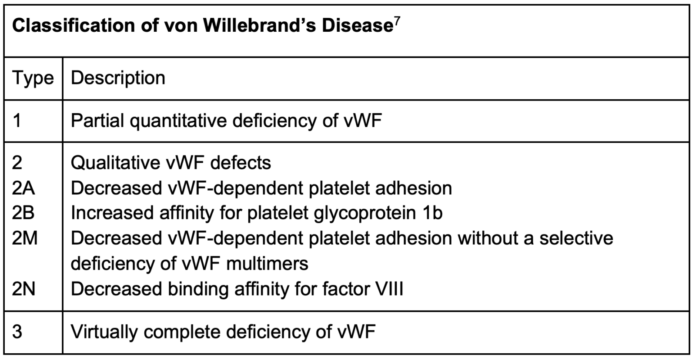
Table 2. Classification of von Willebrand’s (vWF) Disease
Hemophilia
- Hemophilia A (Factor VIII Deficiency) and Hemophilia B (Factor IX Deficiency): These X-linked recessive disorders primarily affect males, but female carriers can experience bleeding symptoms, particularly if they have low factor levels.6
- Management involves replacing the deficient factor. Pregnancy in a carrier necessitates careful monitoring of factor levels and ensuring adequate factor replacement for labor and delivery, especially for invasive procedures.
- Regional anesthesia is contingent on sufficient factor levels to prevent spinal hematoma.
- Factor XI deficiency, though estimated to be homozygous in presentation in the general population at a prevalence of 1:1,000,000, it is heterozygous in presentation in about 8% of the general population. In pregnancy, Factor XI deficiency can pose an increased risk for bleeding that does not necessarily correlate with factor levels. Therefore, a thorough evaluation is recommended.
- Measuring Factor XI levels, obtaining a thorough bleeding history, and referring patients for Hematology consultation are recommended.
- Regional anesthesia is contingent on the appropriate evaluation of Factor XI levels and the absence of a positive bleeding history.6
- The incidence of factor XI deficiency diagnosed during or immediately before pregnancy is increasing due to genetic testing. Patients diagnosed by genetic testing as heterozygous for factor XI deficiency with no clinical signs of bleeding are typically candidates for neuraxial anesthesia.
Assessing Coagulation
- During normal pregnancy, the changes to clotting factors contribute to the shortening of the prothrombin time and activated partial thromboplastin time.5 Routine screening of coagulation studies is helpful in high-risk patients. Viscoelastic testing and platelet function analysis can also be useful tools.
Viscoelastic Testing
- Both thromboelastography and rotational thromboelastometry measure whole-blood coagulation and have been recommended as tools in assessing hemostasis prior to delivery or the initiation of neuraxial anesthesia.
- Parameters measured through viscoelastic testing can help to better isolate portions of the coagulation cascade that may contribute to clotting dysfunction.
References
- Chandra M, Paray AA. Natural physiological changes during pregnancy. Yale J Biol Med. 2024;97(1):85-92. PubMed
- Pavord S, Myers B, Robinson S, Allard S, Strong J, Oppenheimer C; British Committee for Standards in Haematology. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol. 2012;156(5):588-600. PubMed
- Peña-Rosas JP, De-Regil LM, Dowswell T, Viteri FE. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2012. PubMed
- Pishko AM, Marshall AL. Thrombocytopenia in pregnancy. Hematology Am Soc Hematol Educ Program. 2022;2022(1):303-11. PubMed
- Chestnut D, Wong C, Tsen L, et al. Chestnut’s Obstetric Anesthesia: Principles and Practice. 6th Edition, April 2019.
- Gerber GF, Klute KA, Chapin J, et al. Peri- and postpartum management of patients with factor XI deficiency. Clin Appl Thromb Hemost. 2019;25. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.