Copy link
Perioperative Fluid Administration in Children
Last updated: 09/25/2023
Key Points
- Pediatric patients are physiologically more prone to hyponatremia during the perioperative period. Therefore, only isotonic fluids should be used for resuscitation.
- Instead of the 4-2-1 rule, healthy children presenting with marginal to moderate hypovolemia (e.g., fasting for surgery) should be administered 20-40 mL/kg of isotonic fluids during the surgery and postanesthesia care unit.
- Postoperatively, isotonic fluids should be administered using half the 4-2-1 rule (i.e., 2-1-0.5) for the first 12 hours or until the patient starts drinking.
- Unlike in adults, hypotension is a late finding of dehydration in pediatric patients.
- Neonates and infants less than 6 months of age who are fasting for surgery or procedures should be given glucose.
Introduction
- The goal of perioperative fluid administration is to maintain an adequate intravascular volume and avoid fluid overload or hypovolemia. Perioperative patients are prone to hyponatremia secondary to multiple factors, including prehydration with hypotonic fluids, pain, stress, etc., which can lead to the release of antidiuretic hormone (ADH) during and after surgery. Acute hyponatremia can result in cerebral edema and seizures. Younger children are more prone to hyponatremic encephalopathy secondary to their larger brain-to-skull ratio1. Therefore, only isotonic fluids should be administered in the perioperative period.
- In 1957, Holliday and Segar provided calculations for maintenance fluid requirement in children.2 They postulated that the average need for water in hospitalized children in milliliters (mL) parallels energy expenditure in calories (Table 1).
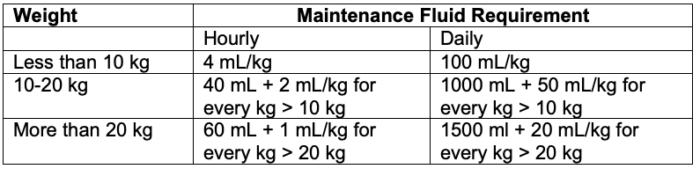
Table 1. Maintenance fluid requirement in children per the 4-2-1 rule
- This formula for maintenance fluid requirement has been further simplified and referred to as the “4-2-1” rule – 4 mL/kg/h for the first 10 kg, 2 mL/kg/h for the next 10 kg, and 1 ml/kg/h for each kg thereafter.3
- Of note, their original approach involved using hyponatremic glucose-containing solutions rather than isotonic solutions. It was meant to be used in children at a basal metabolic state, not acutely ill children or under conditions of stress.
- In a recent commentary, Holliday et al. pointed out several problems with applying the 4-2-1 rule in acutely ill children.4 Increased ADH secretion is common in the perioperative period and can be result from pain, stress, inflammation, surgery, fasting, medications, etc.3 Administration of hypotonic maintenance fluids in this setting can increase the risk of hyponatremia. Instead, a better approach is the rapid expansion of the extracellular fluid with 20-40 mL/kg of isotonic fluids before maintenance fluid therapy is started.4
- Therefore, an approach in healthy children presenting with marginal to moderate hypovolemia (e.g., fasting for surgery) is to administer 20-40 mL/kg of isotonic fluids during the surgery and postanesthesia care unit (as rapidly as 10-20 mL/kg/h).3 Importantly, hypotonic fluids should not be used for resuscitation in the perioperative period.
- If hypovolemia is more severe (e.g., after a bowel preparation), 40-80 mL/kg of isotonic fluids may be necessary during the perioperative period.
- Caution should be used when administering large fluid volumes in the presence of heart disease and renal failure.
- The severity of surgical and nonsurgical trauma should also be considered. This may constitute the largest volume of fluid loss or fluid redistribution, primarily from the extracellular fluid (ECF) compartment.
- In neonates, consider 6-10 mL/kg/hr for intraabdominal and 4-7 mL/kg/hr for intrathoracic surgeries.
- In older infants, consider 6 mL/kg/hr for mild trauma, 8 mL/kg/hr for moderate trauma, and 10 mL/kg/hr for maximal trauma.1
- Blood losses and fluids needed to support systemic blood pressure.
- Blood loss ≥ 20 mL/kg should be replaced with equal volume of packed red blood cells.1
- Postoperatively, intravenous fluid therapy should include an isotonic solution to replace ongoing fluid losses plus half the rate described in the original 4-2-1 fluid regimen, i.e., 2 mL/kg for the first 10 kg, 1 mL/kg for the next 10 kg, and 0.5 mL/kg for each additional kg thereafter for the first 12 hours, until the child starts drinking.3 Again, isotonic fluids should be used instead of hypotonic fluids, such as 0.45% saline.
Glucose Infusion Rate
- Neonates and infants younger than six months of age often require glucose maintenance when fasting due to low glycogen reserves.5,6
- For long surgeries, glucose should be added to the maintenance fluids and titrated to maintain euglycemia.7
- Normal blood glucose for newborns is approximately 40-60 mg/dL.
- Basal glucose requirements are 5-8 mg/kg/min; this is known as glucose infusion rate (GIR). This is most often given as D10.
- To calculate the rate of D10 dextrose infusion to match a target GIR, the following formula can be used: D10 infusion rate (in mL/kg /hr) = (6 x GIR) / 10
- To target a GIR of 5 mg/kg/min, a safe place to start a D10 infusion would be 3 mL/kg/hr.8
- Patients who are born to mothers with diabetes, premature or small for gestational age patients, and patients with processes that are hypermetabolic, like sepsis, should also be given glucose as they are prone to hypoglycemia.9
- Patients on preoperative glucose infusion and total parenteral nutrition should be continued on these infusions to prevent hypoglycemia with glucose checks at regular intervals during the perioperative period.
Isotonic vs. Balanced Salt Solutions
- The composition of plasma and commonly used intravenous fluids is listed in Table 2.
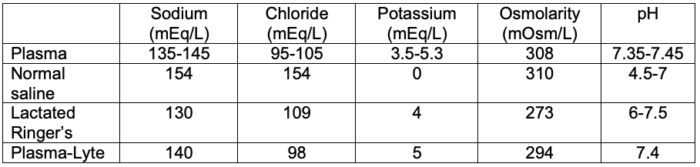
Table 2. Composition of plasma and commonly used intravenous fluids
- Compared to isotonic normal saline, balanced salt solutions such as lactated Ringer’s solution and Plasma-Lyte have varying amounts of a buffering agent, such as lactate, gluconate, or acetate.1 They also have variable amounts of potassium, calcium, and magnesium and a lower amount of sodium.
- Normal saline (Na+ 154 mmol/L) has a higher sodium concentration and osmolarity than plasma but results in normal osmolarity, whereas Plasma-Lyte. (Na+ 140 mmol/L) and lactated Ringer’s solution (Na+ 130 mmol/L) are hypotonic in relation to plasma.1 Therefore, lactated Ringer’s solution should be used with caution in patients at risk for hyponatremia or cerebral edema.
- There is concern that aggressive resuscitation with normal saline can precipitate hyperchloremic metabolic acidosis, renal vasoconstriction, delayed micturition, and acute kidney injury.1
Renal Developmental Physiology
- In early fetal development, the electrolyte regulation and acid-base homeostasis roles of the kidneys are accomplished by the placenta.10,11
- The fetal kidneys contribute significant volume to the amniotic fluid.
- Fetal kidneys begin making urine at about 9 weeks gestational age.1
- An inability to do so can lead to oligohydramnios.
- Nephrogenesis is completed by 35 to 36 weeks gestation.1
- Neonates have a lower glomerular filtration rate (GFR) than adults.
- This is secondary to lower mean arterial pressures and higher vascular resistance.
- Neonates given a water load will excrete it more slowly than adults.
- Neonates are more prone to serum hypoosmolarity when given dilute fluids, which can manifest as hyponatremia and seizures.
- Despite a low GFR, full-term infants can conserve sodium.11
- In contrast, preterm infants have a prolonged glomerulotubular imbalance.
- GFR is high relative to tubular capacity to reabsorb sodium.
- This is due to incomplete development of sodium transport system and poor response to mineralocorticoids in the distal tubule.
- GFR in newborns increases with age and reaches adult levels by around two years of age.10,11
- The kidney’s ability to concentrate urine is lower at birth, especially in premature infants.
Signs and Symptoms of Hypovolemia
- Pediatric patients have higher insensible losses due to relatively increased surface area, increased respiratory rate, and greater metabolic rate, and are therefore, more prone to become hypovolemia in the perioperative setting.
- An inability to communicate thirst to caretakers is also a contributor to relative hypovolemia in the perioperative period.5
- Dehydration can occur very quickly in infants because disorders that result in vomiting or diarrhea can produce deficits of 50 to 100 mL/kg.1
- The clinical signs and symptoms and laboratory assessment of dehydration in children is listed in Table 3. Of note, fluid distribution changes significantly in children older than 2 years. Therefore, using these weight loss measurements as a surrogate of dehydration severity in older children should be avoided.
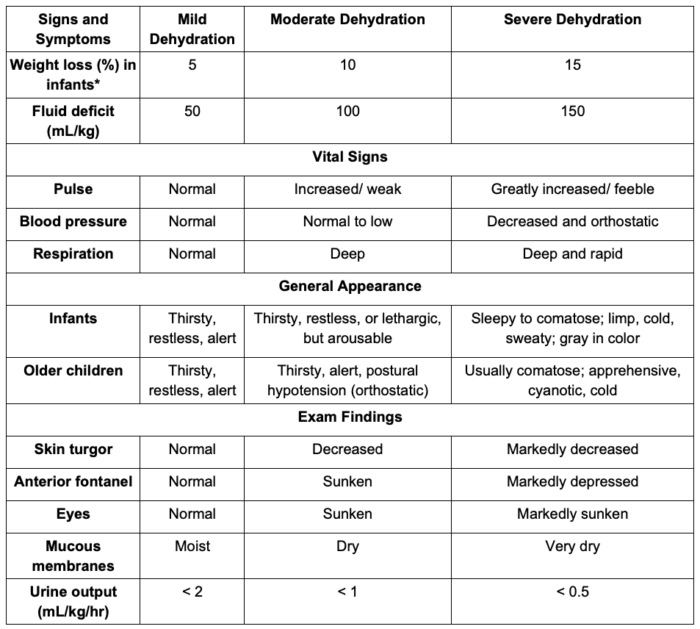
Table 3. Clinical and laboratory assessment of dehydration in children. Adapted from Moritz ML, Ellis D. Regulation of fluids and electrolytes. In: Davis PJ, Cladis FP (Eds). Smith's Anesthesia for Infants and Children. Tenth Edition. Elsevier. 119-157.
Resuscitation
- A mildly dehydrated patient who can take oral fluids should be given 50 mL/kg over 4 hours to replace their deficit.1
- A moderately dehydrated patient who can take oral fluids should be given 100 mL/kg over 4 hours to replace their deficit.
- Deficit can be estimated based on weight loss, and in an admitted patient, can be estimated from measurable output, such as urine output, chest tube drainage, drain output, and emesis.
- Pediatric rehydration solutions should be administered instead of plain water, as these contain carbohydrates and electrolytes that can better maintain homeostasis in the setting of dehydration.
- Patients with severe dehydration should be administered an intravenous fluid bolus of 20-40 mL/kg of a balanced salt solution (lactated Ringer’s solution, or Plasma-Lyte) rapidly and may be repeated if needed, until the patient is hemodynamically stable.1 Hypotonic fluids should be avoided until the patient is adequately resuscitated. Subsequently, an intravenous infusion of the daily maintenance fluid requirement can be administered for the next 24 hours.6
- In the first 8 hours, they should receive half their remaining fluid deficit.
- In the next 16 hours, they should receive the remaining half.
References
- Moritz ML, Ellis D. Regulation of fluids and electrolytes. In: Davis PJ, Cladis FP (Eds). Smith's Anesthesia for Infants and Children. Tenth Edition. Elsevier. 119-157.
- Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics.1957; 19(5): 823-832. PubMed
- McClain CD, McManus ML. Fluid management. In: Cote C, Lerman J, Anderson B (Eds). A Practice of Anesthesia for Infants and Children. Sixth Edition. Elsevier. 2019. 199-216
- Holliday MA, Segar WE, Friedman A, et al. Intravenous fluid therapy for seriously ill children. Lancet. 2004;363(9404):241; author reply 242. PubMed
- Jospe N, Forbes G. Fluids and electrolytes - Clinical aspects. Pediatr Rev. 1996; 17(11): 395-403. PubMed
- Kight BP, Waseem M. (2020). Pediatric fluid management. StatPearls Publishing. PubMed
- Datta P, Aravindan A. (2017). Glucose for Children during Surgery: Pros, Cons, and Protocols: A Postgraduate Educational Review. Anesthesia: Essays and Researches.
- Solimano A. Drop that calculator! You can easily calculate the glucose infusion rate in your head and should! Paediatr Child Health. 2020;25(4):199-200. PubMed
- Gustafsson, J. Neonatal energy substrate production. Indian J Med Res. 2009; 130(5): 618-23. PubMed
- Kelly LK, Seri I. Renal developmental physiology: Relevance to clinical care. Neoreviews. 2008; 9 (4): e150–e161. Link
- Upadhyay KK, Silverstein DM. Renal development: a complex process dependent on inductive interaction. Curr Pediatr Rev. 2014;10(2):107-14. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.