Copy link
Epinephrine and Norepinephrine
Last updated: 09/25/2025
Key Points
- Epinephrine is the first-line vasopressor in the management of cardiac arrest and anaphylaxis. Its mixed α and β effects provide hemodynamic support, bronchodilation, and mast cell stabilization. Adverse effects include tachyarrhythmias, hyperglycemia, and lactic acidosis.
- Norepinephrine is the first-line vasopressor for septic shock due to its potent α1-mediated vasoconstriction with limited β2 activity. Compared to epinephrine and dopamine, it has a lower arrhythmia burden and fewer metabolic derangements.
Introduction and Physicochemical Properties
- Epinephrine and norepinephrine are naturally occurring catecholamines produced by the adrenal medulla. Norepinephrine is synthesized from tyrosine and then methylated to form epinephrine.1
- Epinephrine is the primary catecholamine stored in the adrenal medulla and released during the stress response. Norepinephrine is released from most sympathetic postganglionic nerve terminals, serving as the main neurotransmitter mediating sympathetic tone.
Physiochemical Properties
- Both epinephrine and norepinephrine are highly polar and hydrophilic, resulting in low lipid solubility and poor penetration into the central nervous system.2
- Additionally, they have short plasma half-lives, which necessitate continuous infusion for therapeutic use.
Mechanism of Action and Pharmacokinetics
Epinephrine3
- Epinephrine exerts its effects through adrenergic receptor agonism of α1, α2, β1, and β2 receptors.
- At lower doses, its effects are primarily mediated through β receptors, producing positive inotropy, while at higher doses, α1-mediated vasoconstriction becomes more prominent.
- Epinephrine activates β1 and β2 receptors via Gs protein-coupled pathways, leading to increased intracellular cyclic adenosine monophosphate (cAMP) and calcium.
- The pharmacokinetics of epinephrine reveal a rapid onset with low oral bioavailability due to first-pass metabolism.
- Intravenous administration produces an immediate onset, while intramuscular administration has an onset of five to ten minutes. Endotracheal administration is possible, though absorption is variable and less reliable.
- Plasma steady state is reached within 10 to 15 minutes of continuous infusion.
- Epinephrine is metabolized by monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT) to vanillylmandelic acid (VMA).3
- Clearance is reduced in the elderly due to decreased enzymatic activity, while higher body weight is associated with increased clearance. Epinephrine has an elimination half-life of less than five minutes, with inactive metabolites such as sulfate and glucuronide conjugates eliminated renally.
Norepinephrine4
- Norepinephrine acts as an agonist of α1, α2, and β1 receptors.
- Compared to epinephrine, it produces stronger α1-mediated vasoconstriction, with comparable β1 effects at higher doses and negligible β2 activity.
- Activation of α1 receptors occurs via Gq protein-coupled signaling, leading to phospholipase-C activation, production of inositol triphosphate and diacylglycerol, and subsequent calcium release.
- α2 receptor stimulation inhibits adenylate cyclase via Gi protein coupling, thereby decreasing cAMP and intracellular calcium.
- The pharmacokinetics of norepinephrine exhibit a rapid onset, with steady state achieved within five minutes of intravenous infusion.
- Its half-life is approximately 2.4 minutes, with around 25% being protein-bound, mainly to albumin.
- Norepinephrine is metabolized by MAO and COMT to VMA and eliminated primarily through renal excretion as inactive sulfate and glucuronide conjugates.
Systemic and Adverse Effects2,5,6
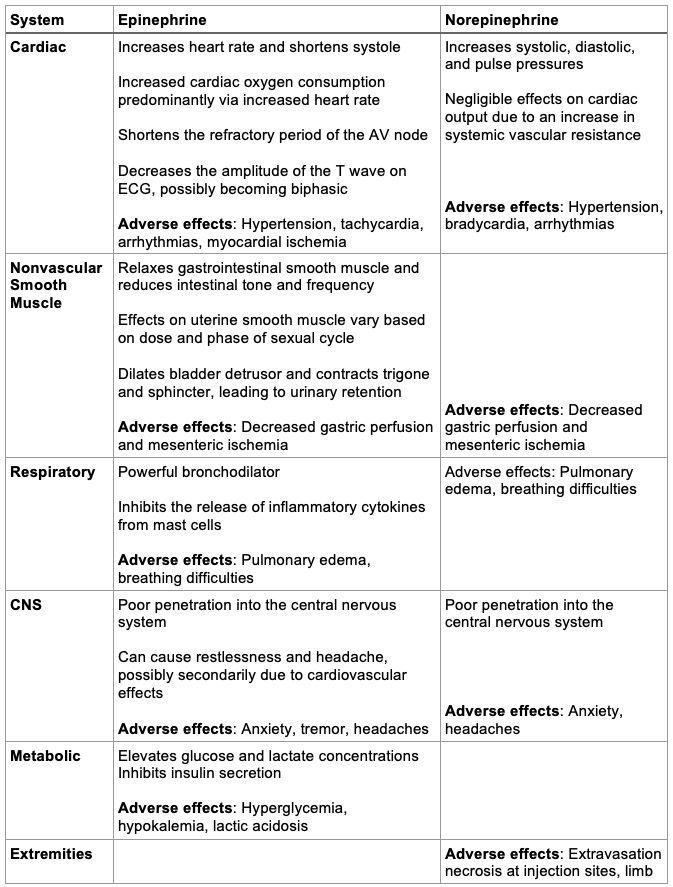
Table 1. Systemic and adverse effects of epinephrine and norepinephrine. Abbreviations: CNS, central nervous system; AV, atrioventricular; ECG, electrocardiogram
Clinical Uses
Epinephrine
- Epinephrine can be administered via intravenous, intramuscular, subcutaneous, nebulized, topical, and endotracheal routes.
- It is the drug of choice for anaphylaxis, providing α-mediated vasoconstriction to raise mean arterial pressure, β-mediated bronchodilation to relieve airway obstruction, and stabilization of mast cells. Doses are given in the mid-anterolateral thigh and can be repeated every 5-15 minutes as needed
- Adult dose: 0.3 – 0.5 mg intramuscular (IM)
- Pediatric dose: 0.01 mg/kg IM, max 0.3mg for children and 0.5mg if >14 years of age
- In cardiac arrest, epinephrine is administered to increase mean arterial pressure and cerebral perfusion pressure during cardiopulmonary resuscitation.
- Adult dose: 1 mg intravenous (IV)/ intraosseous (IO) every 3-5 minutes during cardiopulmonary resuscitation
- Pediatric dose: 0.01mg/kg, max 1mg
- Endotracheal administration is possible, though higher doses of 2-2.5mg are often required due to variable absorption and bioavailability, making IV/IO administration superior and preferable.7
- Additional indications for epinephrine include severe bronchospasm (via β₂-mediated bronchodilation), bradyarrhythmia requiring chronotropic support, cardiogenic shock as an adjunctive inotrope, and as an adjunct to local anesthetics to prolong their duration and limit systemic absorption.
Norepinephrine
- Norepinephrine is limited to intravenous administration, ideally through a central line due to its potent vasoconstrictive effects, though peripheral administration is possible in urgent settings. The recommended maximum dose is 20 mcg/min.8
- It is the first-line vasopressor in septic shock because of its α1-mediated vasoconstriction and lower risk of arrhythmia compared to dopamine.9
- Norepinephrine is also useful in neurogenic shock, cardiogenic shock, and other causes of acute hypotension refractory to fluid resuscitation.
- Adult intravenous dosing typically begins at 0.02 to 0.15 mcg/kg/min, titrated to maintain a target mean arterial pressure of around 65. While no strict maximum dose has been established in dose-response studies, doses above 0.4 mcg/kg/min are associated with significantly increased mortality, and doses exceeding 1.0 mcg/kg/min are predictive of early mortality in critically ill patients.10
- Pediatric dosing is typically 0.05 to 0.1 mcg/kg/min, with consensus recommendations limiting maximum doses to 2.0 mcg/kg/min to minimize adverse effects.11
References
- Barrett KE, Barman SM, Brooks HL, Yuan JXJ. The Adrenal Medulla; Adrenal Cortex. Ganong's Review of Medical Physiology, 27th Edition. McGraw Hill; 2025.
- Zimmerman J, Lee JP, Cahalan M. 25 - Vasopressors and Inotropes. In: Hemmings HC, Egan TD, eds. Pharmacology and Physiology for Anesthesia (Second Edition). Elsevier; 2019:520-534.
- DailyMed. ADRENALIN (epinephrine in sodium chloride) injection label. DailyMed website. Published May 20, 2025. Accessed July 6, 2025. Link
- DailyMed. LEVOPHED (norepinephrine bitartrate) injection label. DailyMed website. Drug label information updated June 12, 2025. Accessed July 6, 2025. Link
- Tilley DG, Houser SR, Koch WJ. Adrenergic Agonists and Antagonists. In: Brunton LL, Knollmann BC, eds. Goodman & Gilman's: The Pharmacological Basis of Therapeutics, 14th Edition. McGraw-Hill Education; 2023.
- Levy B, Perez P, Perny J, Thivilier C, Gerard A. Comparison of norepinephrine-dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Crit Care Med. 2011;39(3):450-5. PubMed
- Manisterski Y, Vaknin Z, Ben-Abraham R, et al. Endotracheal epinephrine: a call for larger doses. Anesth Analg. 2002;95(4):1037-41. PubMed
- Abu Sardaneh A, Penm J, Oliver M, et al. International pharmacy survey of peripheral vasopressor infusions in critical care (INFUSE). J Crit Care. 2023;78:154376. PubMed
- Backer DD, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779-789. PubMed
- Martin C, Medam S, Antonini F, et al. Norepinephrine: Not too much, too long. Shock. 2015;44(4):305-9. PubMed
- Oualha M, Tréluyer JM, Lesage F, et al. Population pharmacokinetics and haemodynamic effects of norepinephrine in hypotensive critically ill children. Br J Clin Pharmacol. 2014;78(4):886-97. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.