Copy link
Environmental Impact of Nitrous Oxide
Last updated: 05/10/2023
Key Points
- Nitrous oxide (N2O) is a potent greenhouse gas and the leading cause of ozone depletion worldwide.
- With sevoflurane and isoflurane, the use of nitrous oxide to carry the volatile agent (to decrease the concentration of agent needed while maintaining minimum alveolar concentrations) results in net higher total greenhouse gas emissions than higher concentration volatile agents carried in oxygen.
- Most purchased nitrous oxide is lost to the environment due to leaks from centralized storage tanks and its piped transport to anesthesia machines.
Nitrous Oxide: Greenhouse gas and Ozone Depletion Agent
- Global warming potential (GWP) is a chemical property that describes the ability of a gas to trap heat in the atmosphere. Volatile anesthetics and nitrous oxide are far more potent greenhouse gases (GHG) than CO2 and methane as shown in Table 1.1
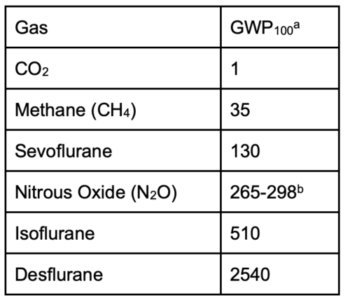
Table 1. GWP of anesthetic gases compared to common greenhouse gases. a- GWP100 refers to impact over a 100-year time period. While GWP20 can also be used, GWP100 is most commonly used in the anesthesia sustainability literature.
b- GWP100 of N2O varies in the published literature.
- In addition to contributing to global warming as a GHG, nitrous oxide also destroys ozone. Nitrous oxide persists in the atmosphere for 114 years and is the “single greatest ozone-depleting substance” emitted since chlorofluorocarbons (CFCs) became heavily regulated in the late 1980s and early 1990s following the Montreal Protocol on Substances that Deplete the Ozone Layer.2
- While the use of nitrous oxide as the carrier gas for volatile anesthetics does allow for reduced volatile agent concentration, this only lessens the environmental impact if desflurane, by far the most environmentally harmful volatile agent, is being used. The GHG emissions of an anesthetic that includes nitrous oxide were summarized by Sherman3 as shown in Figure 1 which shows the emissions generated by a 1-MAC anesthetic comprised of 37% volatile agent and 63% nitrous oxide. For sevoflurane and isoflurane the volatile anesthetic GHG emissions spared by using nitrous oxide are eclipsed by the GHG emissions from the nitrous oxide itself. For desflurane, the emissions generated by using nitrous oxide carrier to provide 63% MAC are less than the emissions that would be generated from 1-MAC desflurane in oxygen.
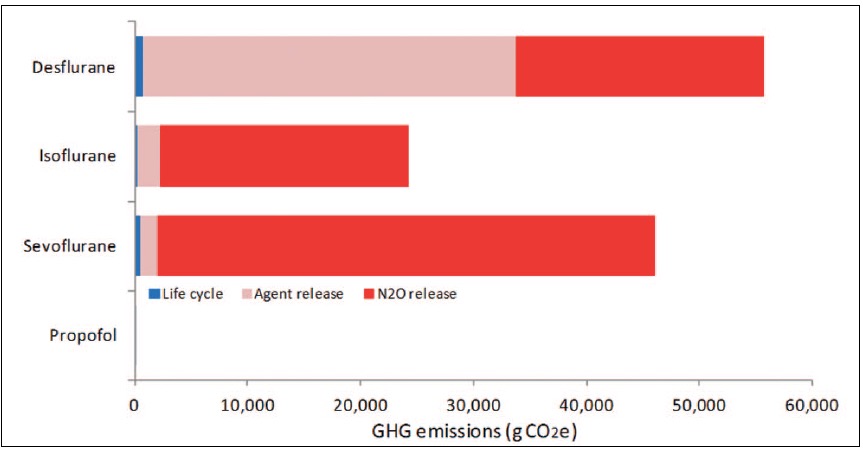
Figure 1. Life cycle GHG emissions of anesthetics co-administered with nitrous oxide. These calculations assume nitrous oxide and oxygen in a 60%/40% mix as the carrier gas and volatile agents providing 37% of MAC while nitrous oxide provides 63% of MAC. Desflurane and isoflurane at 1 Lpm fresh gas flows (FGF), sevoflurane at 2 Lpm FGF. Adapted from Sherman J, et al. Life cycle greenhouse gas emissions of anesthetic drugs. Anesth Analg. 2012.3
Nitrous Oxide Storage and Unintended Leakage
- Nitrous oxide can be stored in three main ways in the hospital environment (Table 2).
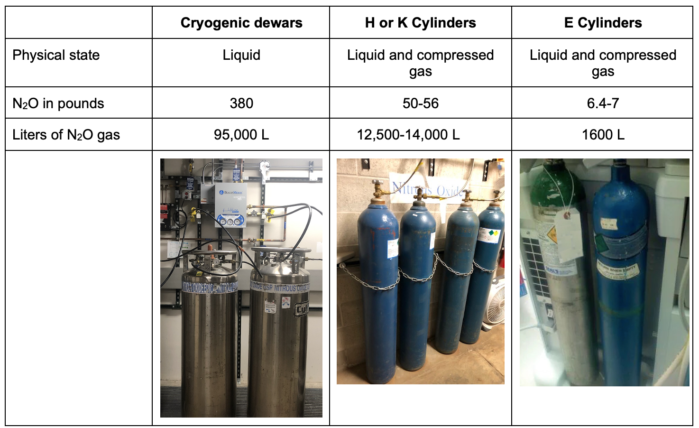
Table 2. Options for storage of nitrous oxide in the hospital setting.
- Nitrous oxide in cryogenic dewars and H or K cylinders is usually stored centrally with other medical gases and piped to anesthesia machines.
- Nitrous oxide in E cylinders is stored and used at the anesthesia machine.
- Until recently, the amount of nitrous oxide that was bought was assumed to be what was used clinically. However, recent studies comparing clinical usage to procurement found that over 95% of centralized stores of liquid and compressed gas nitrous oxide is actually leaked from the tanks themselves or through the pipelines that lead to anesthesia machines.4
- Nitrous oxide in cryogenic tanks continually warms (boiling point of -88℃) and vaporizes over time. As the pressure within the tank increases and reaches 350 PSIG, it will be released from the pressure relief valves located on the dewars. Nitrous oxide in H, K, and E cylinders is stored in high pressure cylinders without pressure relief valves.
- Additionally, as nitrous oxide is under high pressure throughout the system, every connection point from the centralized tanks to the pipeline connection sites and finally to the anesthesia machine is a potential leakage risk.
- This leakage is inherent to the storage of nitrous oxide as a liquid in tanks with pressure relief valves and by piping it long distances throughout hospitals to anesthetizing locations.
- Leaked nitrous oxide may have health implications, as the National Institute for Occupational Safety and Health has set exposure rates of less than 25 ppm. In addition, this is a poor use of material and financial resources.
- It has been shown that using E cylinders at the anesthesia machine and keeping the tanks closed except during use essentially eliminates the leaked fraction of purchased nitrous oxide.5
- There is a movement in sustainable anesthesia to decommission centralized nitrous oxide storage and piping or to not include centrally sourced nitrous oxide in new builds.
References
- Sulbaek Andersen MP, Nielsen OJ, Wallington TJ, et al. Assessing the impact on global climate from general anesthetic gases. Anesth Analg. 2012; 114:1081–5. PubMed
- Ravishankara AR, Daniel JS, Portmann RW. Nitrous Oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science. 2009;326 (5949):123-5. PubMed
- Sherman J, Le C, Lamers V, et al. Life cycle greenhouse gas emissions of anesthetic drugs. Anesth Analg. 2012; 114:1086–90. PubMed
- Seglenieks R, Wong A, Pearson F, et al. Discrepancy between procurement and clinical use of nitrous oxide: Waste not, want not. Br J Anaesth. 2022;128(1): e32-34. PubMed
- Herr M, Outterson R, Aggarwal S. Lost in the ether: The environmental impact of anesthesia. Operative Techniques in Orthopaedics. 2022;32(4):100997. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.