Copy link
Traditional Tests of Coagulation and Fibrinolysis
Last updated: 10/11/2023
Key Points
- The prothrombin time (PT) measures the time it takes plasma to clot when exposed to tissue factor and it assesses the extrinsic and common pathways of coagulation.
- The activated partial thromboplastin time (aPTT) measures the time it takes plasma to clot when exposed to substance that activate the contact factors coagulation and it assesses the intrinsic and common pathways of coagulation.
- Different assays can be used to monitor anticoagulation therapy.
Introduction
- The traditional coagulation tests include PT, aPTT, international normalized ratio (INR), thrombin time (TT), fibrinogen levels, activated clotting time (ACT), etc.
- Coagulation tests can be broadly divided into clot-based and chromogenic assays.1 In both techniques, whole-blood samples are centrifuged to obtain platelet-poor plasma. Test specific reagents are then added.
- In clot-based assays (such as PT, aPTT, and TT), the time to fibrin formation is detected by mechanical, turbidimetric, or other means, and compared to a standard normogram.1
- Chromogenic assays use specific factor substrates bound to a chromophore and release a colored compound when cleaved proportionally to the amount of factor present.1 Chromogenic assays are used to measure coagulation factors VIII, IX, X, XIII, antithrombin, plasminogen, protein C, etc.
- The traditional fibrinolysis tests include fibrin degradation products and D-dimer levels.
- Point-of-care viscoelastic testing will be covered in a separate summary.
Prothrombin Time (PT)
- PT measures the time it takes plasma to clot when exposed to thromboplastin (a mixture of tissue factor, calcium, and phospholipid). It assesses the extrinsic and common coagulation pathways (Figure 1).2,3
- The normal ranges for PT varies based on the laboratory and reagent/instrument combination. Local institutional ranges should be used while interpreting PT. The normal range for most laboratories is approximately 11-13 seconds.2
- Low levels of factors VII, X, and V, prothrombin, Vitamin K and fibrinogen prolong the PT.
- Common indications for evaluating PT include3
- Monitoring patients on warfarin therapy
- Evaluating a bleeding patient
- Diagnosing disseminated intravascular coagulation (DIC)
- Assessing liver function and to calculate the model for end-stage liver disease score in patients with advanced liver disease
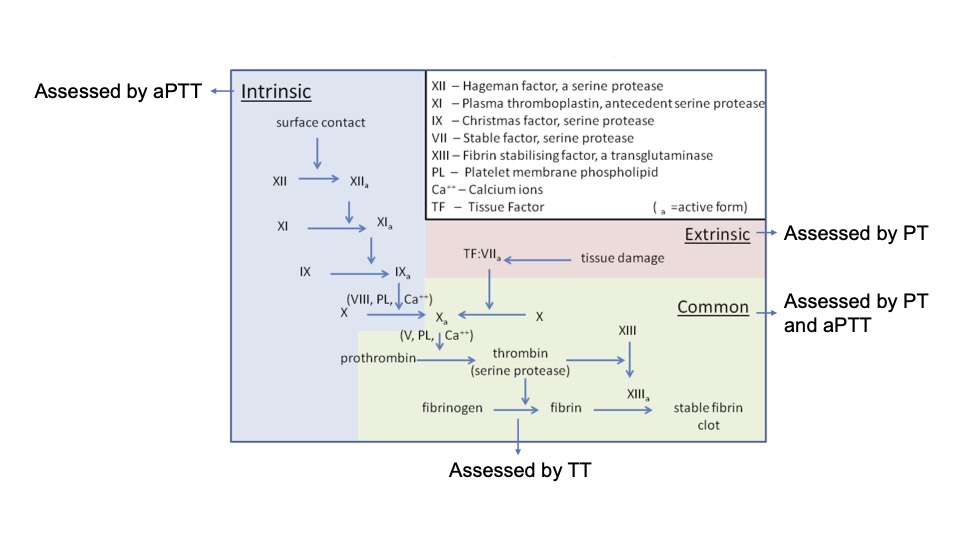
Figure 1. Schematic representation of coagulation pathway and coagulation tests that assess each pathway.
Abbreviations: aPTT, activated partial thromboplastin time; PT, prothrombin time; TT, thrombin time
Adapted from Wikimedia Commons. Dr. Graham Beards. CC BY SA 3.0 Link.
International Normalized Ratio (INR)
- The INR is calculated as a ratio of the patient’s PT to a control PT obtained using a World Health Organization (WHO)-approved thromboplastin reagent.2,4
- INR = (Patient PT ➗ Control PT).ISI ISI refers to International Sensitivity Index which depends on the reagent used.
- The INR will be similar on a blood sample tested in any laboratory and allows comparison of the patient’s tests performed at different times and/or locations.
- The INR is dimensionless. In normal patients who are not on anticoagulation, the INR is 1. For patients on anticoagulant therapy, the therapeutic INR ranges from 2.0 to 3.0.2,4
- The INR was developed to monitor patients on warfarin therapy. It is also commonly used as a surrogate for PT in bleeding patients, to diagnose DIC, and to assess patients with end-stage liver disease.2,4
Activated Partial Thromboplastin Time (aPTT)
- The aPTT measures the time it takes plasma to clot when exposed to substances that activate the contact factors. It assesses the intrinsic and common coagulation pathways.2,5
- The normal range for the aPTT varies by laboratory and reagent/instrument combination. Local institutional ranges should be used while interpreting aPTT. The normal range for most laboratories is approximately 25-35 seconds.2
- Low levels of factors VIII, IX, XI, and XII prolong the aPTT. Adequate levels of factor X, V, prothrombin, and fibrinogen must also be present for a normal aPTT.
- The aPTT is commonly used to monitor heparin therapy, to target therapeutic anticoagulation, and as a part of a “coagulation panel” to evaluate a bleeding patient.5
Thrombin Time (TT)
- Also known as thrombin clotting time, TT is a measure of the ability of thrombin to convert fibrinogen to fibrin. TT involves adding thrombin from human or bovine sources to platelet-poor plasma and measuring the time to fibrin clot formation.1,2
- The normal TT is less than 30 seconds (usually between 14-19 seconds).2
- TT may be prolonged if the fibrinogen levels are low (hypofibrinogenemia), dysfunctional fibrinogen (dysfibrinogenemia), or if an anticoagulant that inhibits thrombin is present in the sample.
Fibrinogen Levels
- Fibrinogen, a glycoprotein synthesized by the liver, is the major structural component of a clot. Normal fibrinogen levels range between 160 and 350 mg/dL. When fibrinogen levels are below 100mg/dL, clot production may not be possible.
- Fibrinogen disorders can be classified as6
- Afibrinogenemia: an absence of circulating fibrinogen
- Hypofibrinogenemia: reduced levels of circulating fibrinogen
- Dysfibrinogenemia: circulating fibrinogen is dysfunctional
- Conditions like DIC rapidly deplete fibrinogen levels.
- Conditions like trauma or surgery rapidly increase fibrinogen levels.
Activated Clotting Time (ACT)
- ACT is the most used test for monitoring adequacy of anticoagulation in the operating room, because of its speed, low cost, simplicity, and ability to monitor anticoagulation when large doses of heparin are used.
- ACT measures the time it takes whole blood (not centrifuged plasma like the other traditional tests) to clot when exposed to substances that activate the contact factors. Commonly celite or kaolin, is added to freshly drawn whole blood and the time to clot formation is measured.2
- Like the aPTT, the ACT assesses the intrinsic and common pathways of coagulation.2
- The normal range of ACT is 107 +/- 13 seconds (mean +/- SD).
- ACT measures both the intrinsic and common pathways of coagulation.
- The most common causes of prolonged ACT are hemodilution, hypothermia, thrombocytopenia, and severe platelet dysfunction (once operator error has been ruled out).
- Aprotinin may dramatically and artifactually alter the ACT, depending on the ACT monitoring system used, and may lead to an insufficient heparin dose if this artifact is not taken into consideration.
Anti-Xa Assay
- The anti-Xa assay is a functional chromogenic assay that measures the inhibitory activity against factor Xa in platelet-poor plasma.1
- It is increasingly being used to monitor anticoagulation with unfractionated heparin (which has both anti-Xa and anti-IIa activity) or low-molecular weight heparin (which has primarily anti-Xa activity).1
- For the test, platelet-poor plasma is incubated with a fixed amount of exogenous factor Xa and then residual factor Xa activity is measured using a factor Xa-specific chromogenic substrate. The result is compared to a standard curve generated using dilutions of the specific anticoagulant and normal plasma.
- Similar to monitoring heparin, drug specific anti-Xa tests can be used to measure fondaparinux, apixaban, or rivaroxaban.1
Fibrin Degradation Products (FDP)
- Tests that measure FDPs identify the breakdown of products of fibrin and fibrinogen specifically.
- FDPs will increase in any state of accelerated fibrinolysis such as advanced liver disease, DIC, or in situations following administration of exogenous thrombolytics.
Fibrin D-dimer Levels
- D-dimer assays are specific for the breakdown products of crosslinked fibrin of a mature thrombus, classically seen in pulmonary emboli, deep venous thrombosis, and DIC.2
- Various conditions can cause an elevated D-dimer levels such as a thromboembolism, inflammation (such as in sepsis or COVID-19), liver disease, kidney disease, surgery, trauma, malignancy and/or pregnancy including others.
Monitoring Anticoagulation
- Assays commonly used to monitor anticoagulation are listed in Table 1.
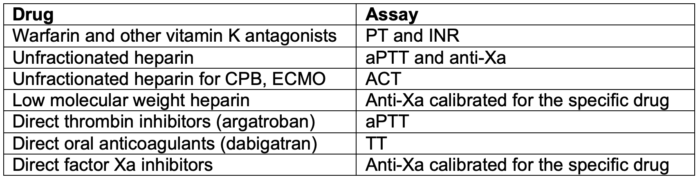
Table 1. Common assays used to monitor anticoagulation.
Abbreviations: CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; aPTT, activated partial thromboplastin time; PT, prothrombin time; TT, thrombin time
Causes of Abnormal Coagulation Studies
- Abnormal coagulation studies in different disease states and factor deficiencies are listed in Tables 2 and 3.
- Warfarin typically prolongs the PT alone, while heparin typically prolongs the aPTT alone. At high levels, heparin can prolong both tests.
- Direct thrombin inhibitors (argatroban, dabigatran) typically prolongs both tests, but at low levels, dabigatran may not prolong the PT.
- Direct factor Xa inhibitors (apixaban, edoxaban, rivaroxaban) can prolong the PT and aPTT.
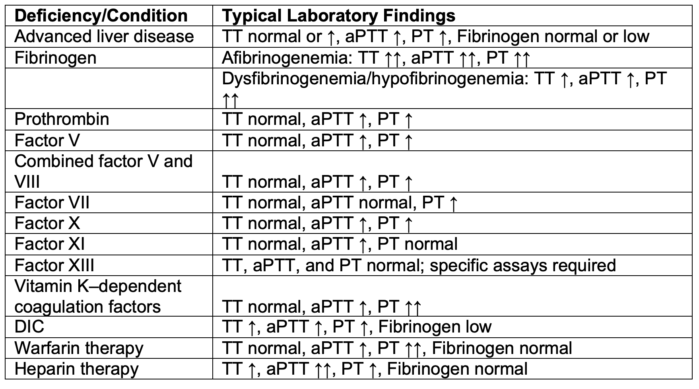
Table 2. Coagulation test abnormalities.
Abbreviations: aPTT, activated partial thromboplastin time; PT, prothrombin time; TT, thrombin time; DIC, disseminated intravascular coagulation
Adapted from Shafer AI. Approach to the patient with bleeding and thrombosis. In: Goldman L, Schafer AI (eds). Goldman-Cecil Medicine, 26th Edition. Elsevier, Philadelphia, PA. 2020.162,1118-1123.e2.7
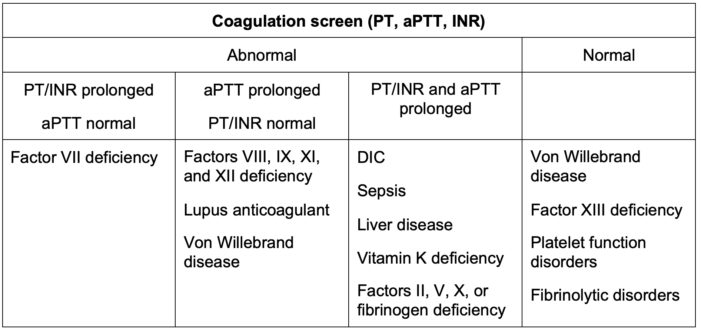
Table 3. Algorithm for the diagnosis of bleeding disorders based on results of a coagulation screen.
Abbreviations: aPTT, activated partial thromboplastin time; PT, prothrombin time; TT, thrombin time; DIC, disseminated intravascular coagulation; INR, International Normalized Ratio
Adapted from O’Brien S. Approach to the child with bleeding symptoms. In: Post T(ed) UpToDate. 2023.8
References
- Maier CL, Sneicinski RM. Anticoagulation monitoring for perioperative physicians. Anesthesiology. 2021; 135: 738-48. PubMed
- Zehnder JL. Clinical use of coagulation tests. In: Post T (ed). UpToDate; 2023. Accessed May 16th, 2023. Link
- Yang R, Moosavi L. Prothrombin time. In: StatPearls (Internet). Treasure Island, FL. StatPearls Publishing. 2023. Link
- Shikdar S, Vashist R, Bhattacharya PT. International normalized ratio. In: StatPearls (Internet). Treasure Island, FL. StatPearls Publishing. 2023. PubMed
- Rountree KM, Yaker Z, Lopex PP. Partial thromboplastin time. In: StatPearls (Internet). Treasure Island, FL. StatPearls Publishing. 2023. PubMed
- Kaur J, Jain A. Fibrinogen. In: StatPearls (Internet). Treasure Island, FL. StatPearls Publishing. 2023. Link
- Schafer AI. Approach to the patient with bleeding and thrombosis. In: Goldman L, Schafer AI (eds). Goldman-Cecil Medicine, 26th Edition. Elsevier, Philadelphia, PA. 2020. 162, 1118-1123.e2.
- O’Brien S. Approach to the child with bleeding symptoms. In: Post T(ed). UpToDate. 2023. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.