Copy link
Eutectic Mixture of Local Anesthetic
Last updated: 07/29/2025
Key Points
- Eutectic mixture of local anesthetic (EMLA) contains prilocaine and lidocaine and is an effective option for local analgesia that can be applied directly to the skin without the need for vascular access.
- The major limiting factor for its use is the time to onset (ranging from 30 minutes to 2 hours) of analgesia after application.
- EMLA has several safety concerns and should be avoided in patients at risk for methemoglobinemia, such as G6PD deficiency or the concurrent use of methemoglobin-inducing medications.
Introduction
- EMLA cream is a mixture of 2.5% prilocaine and 2.5% lidocaine. Both are amide local anesthetics.1
- The word “eutectic” refers to a combination of two drugs that, when combined, result in a final product with a lower melting temperature than either of the two drugs alone, thereby contributing to its effectiveness.2,3
- EMLA was created to overcome the barrier of intact skin, not present in oral or tracheal mucosa, where other common local anesthetics may be used.1
- EMLA works by releasing the compound through the skin barrier, which collects within the dermal layer of skin to directly act upon nerve endings and pain receptors.
- The mixture is available in both paste and cream forms.
- Historically, a patch form of EMLA was available, but it is no longer available in the United States.
- EMLA has been established as a safe and effective analgesic modality.
Drug Properties
- EMLA most commonly provides a depth of analgesia reaching up to 0.5cm below the skin due to the ability to penetrate the stratum corneum, unlike many other local anesthetics.1
- Time to onset of full analgesia from application varies from 30 minutes to 2 hours or more.
- Standard doses include 1-2g of cream applied over a 10cm2 area of skin.1 The dosing of EMLA in children is listed in Table 1.
- Recommendations for ensuring full effect include covering the area with an occlusive dressing after application and allowing a time window of up to 1 hour before any procedure.1
- The above relies heavily on application time, amount of dermal blood flow, and total dose given.1
- The skin may be numb for 1-2 hours after the cream is removed.
- Each gram of EMLA contains 25 mg of lidocaine, 25mg of prilocaine, polyoxyethylene fatty acids (as emulsifiers), carboxypolymethylene (as a thickening agent), sodium hydroxide, and purified water.2
- EMLA cream tubes are available in 5-gram or 30-gram sizes.
- EMLA cream should be stored at 20-25 °C (68-77°F).2
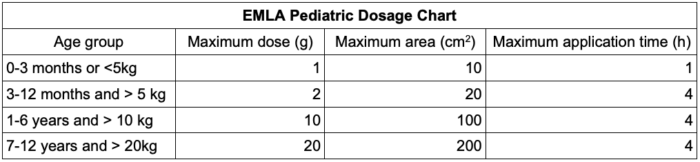
Table 1. EMLA dosing in children2
Clinical Use
- EMLA can be used as a topical anesthetic for use on normal intact skin and genital mucous membranes.2
- MLA can be used to provide analgesia prior to insertion of intravenous (IV) cannulas,1 Port-a-Cath puncture,3 or prior to other needle insertions, including possibly vaccinations.4
- The formulation has also been used in cases of herpes zoster to reduce pain associated with capsaicin treatments.1
- EMLA has been shown to reduce pain in patient’s posthemorrhoidectomy and subsequently leads to a decrease in overall pain medication requirements.5
- Additional reported uses include sharp wound debridement, burn injuries,6 procedures requiring dermal penetration, postcircumcision, repair of perineal trauma, and analgesia for thyroid fine-needle aspiration.5
- Off label use of EMLA for burns has been reported in case reports and case series, although it is only officially approved in intact normal skin.6
- If used off label, use caution, and data suggest that 5 grams of EMLA applier to smaller burns the size of 25cm2 is safe and effective in adults.6
- There is potential for anti-inflammatory effects and improvement of tissue perfusion in burn victims.6
Safety Concerns
- EMLA should not be used on mucous membranes or skin that is broken.1
- If used on a burn wound, the absorption of local anesthetic is much higher with quicker onset.6
- EMLA has been proven to be ototoxic and should not be applied in areas where there is a possibility of contact with the tympanic membrane.7
- EMLA can be used on genital membranes with close monitoring, and the onset is significantly faster due to its more rapid absorption.7,9
- Close monitoring is recommended before, during, and after use in small children due to increased risk of prilocaine-induced methemoglobinemia, especially when higher doses are used for larger burn areas.2,8
- Careful dosing should be practiced in those more sensitive to systemic effects, including acutely ill, debilitated, or elderly, or those with liver disease.
- Common but less concerning side effects include localized skin blanching and localized erythema.4
- In patients with atopic dermatitis, caution should be exercised, as rapid and increased absorption may occur.
- Careful attention should be used in cases where repeated applications of EMLA may be needed, as cumulative concentrations can also lead to methemoglobin formation.
- Signs and complications of methemoglobinemia include cyanosis, desaturation to 85%, seizures, and increased measured concentrations of methemoglobin outside of the normal 1-2% range.
- The treatment for methemoglobinemia includes the administration of methylene blue.2
- Second-line treatment may include benzodiazepines for seizures, exchange transfusions, Vitamin C, and an appropriate form of oxygenation, at times requiring hyperbaric oxygen.2
- Please see the OA summary on methemoglobinemia for more details. Link
Contraindications
- EMLA should not be used in children younger than 37 weeks gestational age and use extreme caution if weighing less than 5 kg.2,8
- EMLA in patients at increased risk for methemoglobinemia should be avoided, including those with a G6PD deficiency and those taking oxidizing medications such as antimalarials and sulfonamides2,7 (Table 2).
- EMLA in patients with congenital or idiopathic methemoglobinemia should be avoided.
- Placement in patient presentations requiring ongoing analgesia should be avoided due to the risk for accumulation toxicity.
- Placement in patients with compromised or damaged skin barrier or an open wound should be avoided.
- EMLA in patients with allergy to amide anesthetics (lidocaine, bupivacaine, ropivacaine, mepivacaine) should be avoided.2
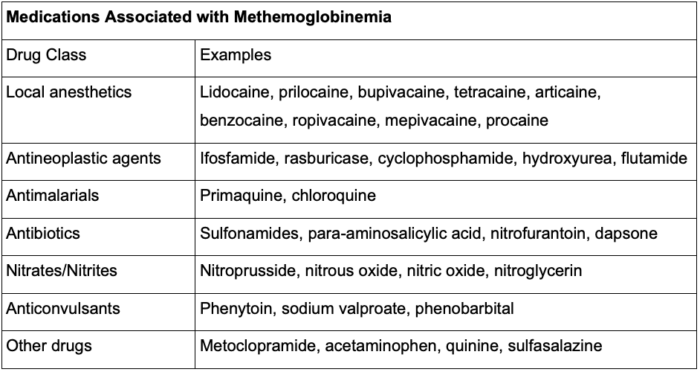
Table 2. Medications associated with methemoglobinemia
Limitations
- In cases of multiple attempts for preanalgesia for IV cannulas, the inability to always accurately predict where an IV site may be needed or successfully placed, and therefore allow adequate time for effect, is a limitation of EMLA.
- For a full analgesic effect, the site of application must be covered with an occlusive dressing and relatively undisturbed, which may not be feasible depending on the patient’s presentation.1
- EMLA is not optimal for patients requiring rapid-onset analgesia.
- EMLA should not be used when the total body surface area requiring analgesia exceeds the maximum safety allowance.
- EMLA should be used with caution in patients on class 1 antiarrhythmic drugs (tocainide and mexiletine), as toxic effects could be synergistic or additive.2
- EMLA should be used with caution in patients on class III antiarrhythmic drugs (sotalol, amiodarone).
- If the patient is already on a drug that can cause methemoglobinemia, they are at an increased risk of toxicity if administered prilocaine or EMLA.
References
- In: Butterworth IV JF, Mackey DC, Wasnick JD. eds. Morgan & Mikhail’s Clinical Anesthesiology, 7e. McGraw-Hill Education; 2022. Accessed July 06, 2025. Link
- U.S. Food and Drug Administration. EMLA (lidocaine 2.5% and prilocaine 2.5%) cream prescribing information. Silver Spring, MD: FDA; 2018. Accessed July 13, 2025. Link
- Lüllmann B, Leonhardt J, Metzelder M, Hoy L, Gerr H, Linderkamp C, Klein C, Grigull L. Pain reduction in children during port-à-cath catheter puncture using local anaesthesia with EMLA™. Eur J Pediatr. 2010;169(12):1465-9. PubMed
- Olsson Duse B, Sporrong Y, Bartocci M, Skoglund K. Efficacy of topical lidocaine-prilocaine (EMLA®) for management of infant pain during pneumococcal vaccination: A randomized controlled trial. Paediatr Neonatal Pain. 2021;4(2):53-60. PubMed
- Al Awadhi K, Allafi FA, Almukaimi BA, et al. Analgesic efficacy of EMLA cream among patients undergoing hemorrhoidectomy: A systematic review and meta-analysis of randomized controlled trials. Cureus. 2024;16(8):e66423. PubMed
- Rangatchew F, Schoelzer L, Drzewiecki KT, Holmgaard R. EMLA cream in burns: A systematic review of safety, analgesic efficacy, and effects on burn pathophysiology. J Plast Reconstr Aesthet Surg. 2024; 95:386-401. PubMed
- Kwatra SG, Loss M. Other Topical Medications. In: Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ, Orringer JS. eds. Fitzpatrick's Dermatology, 9e. McGraw-Hill Education; 2019. Accessed July 06, 2025.
- Harvey DT, Harvey E. Local anesthesia, regional nerve blocks, and postoperative pain management. In: Kantor J. eds. Dermatologic Surgery. McGraw-Hill Education; 2018. Accessed July 07, 2025. Link
- Ibrahim MM, Largent-Milnes TM. Local Anesthetics. In: Vanderah TW. eds. Katzung’s Basic & Clinical Pharmacology, 16th Edition. McGraw-Hill; 2024. Accessed July 07, 2025. Link
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.