Copy link
Ductus Arteriosus Dependency in Congenital Heart Disease
Last updated: 03/11/2024
Key Points
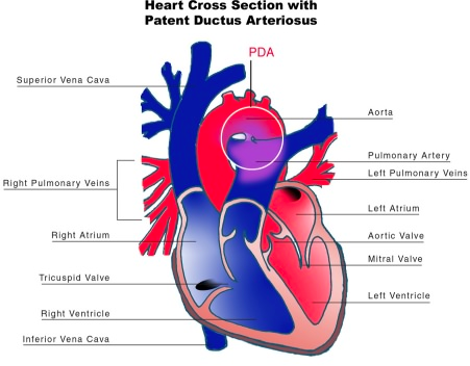
- The ductus arteriosus (DA) is a vascular structure connecting the pulmonary trunk and proximal descending aorta. It is an essential component of the fetal circulation. Shortly after birth, the DA begins to close. It is permanently closed within 2-3 weeks of life in most term neonates.
- A patent DA is necessary for survival in certain congenital heart disease (CHD) lesions.
- Ductal patency can be maintained with the infusion of prostaglandin E1 (PGE1 or Alprostadil) until palliative or corrective surgery can be performed.
Introduction
- The DA is a fetal shunt that allows systemic venous return to bypass the high-resistance pulmonary vascular beds in utero. Certain CHD lesions require a patent DA for survival.
- Ductal-dependent CHD lesions can be grouped into three general groups:
- ductal-dependent pulmonary circulation;
- ductal-dependent systemic circulation; and
- parallel circulation.
- Examples of these lesions are shown in Table 1.
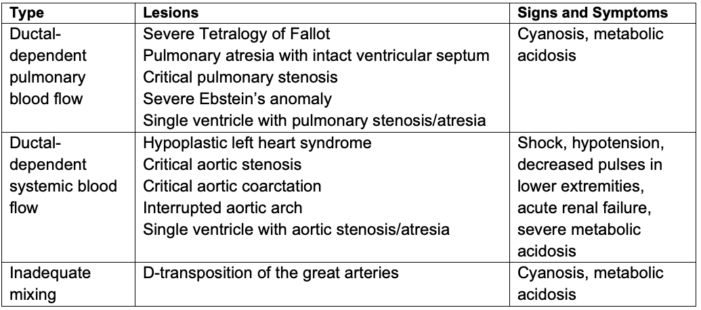
Table 1. Ductal-dependent CHD lesions
Anatomy and Physiology
Anatomy of the Ductus Arteriosus
- During normal development, the distal portion of the left 6th arch persists as the DA, connecting the main pulmonary trunk with the left dorsal aorta, about 5-10 mm distal to the origin of the left subclavian artery.
- The DA is typically conical in shape with large aortic and tapered pulmonary ends. The size of the DA determines the degree of shunting.
Normal Closure of the Ductus Arteriosus
- In term neonates, closure of the DA after birth occurs in two phases:
- Functional closure: When a neonate starts to take breaths after delivery, an increase in arterial PO2, a decrease in pulmonary vascular resistance (PVR), and a drop in circulating PGE2 promote ductus wall constriction and functional ductal closure within 18-24 hours of life.
- Permanent anatomic closure: following functional closure, there is extensive remodeling of the intima and endothelial proliferation of the ductal wall. The process takes 2-3 weeks in most normal full-term neonates and results in the formation of the ligamentum arteriosum.
- Failure of the DA to close results in a patent DA. Please see the OA summary on patent DA for more details. Link
Ductal-Dependent Lesions and Physiology
Ductal-Dependent Pulmonary Circulation
- It is caused by severe obstruction to or restriction of pulmonary blood flow.
- The pulmonary circulation is supplied by the patent DA (Figure 2).
- Postnatal constriction/closure of the DA causes severe hypoxemia and metabolic acidosis. Rapid deterioration and death ensue without intervention.
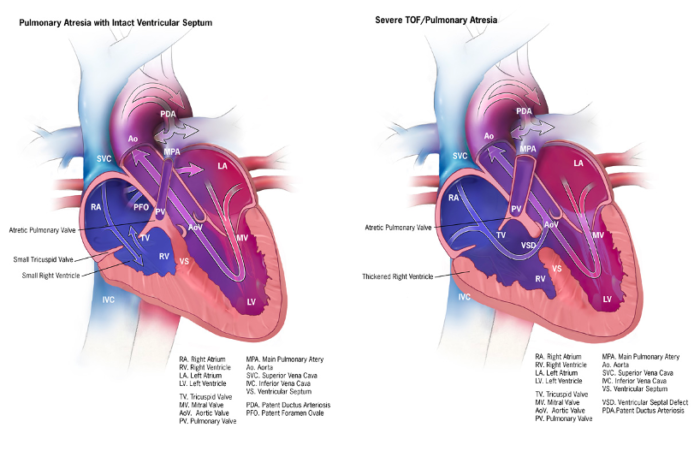
Figure 2. Ductal-dependent pulmonary circulation: Pulmonary atresia with intact ventricular septum and severe tetralogy of Fallot/pulmonary atresia. Source: Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities. Public Domain.
Ductal-Dependent Systemic Circulation
- It is caused by severe obstruction to systemic blood flow.
- Systemic perfusion beyond the level of obstruction is supplied by the DA (Figure 3).
- Postnatal constriction or closure of the DA may cause shock-like presentations, such as systemic hypotension, poor perfusion, severe metabolic acidosis, major organ failure, and death.
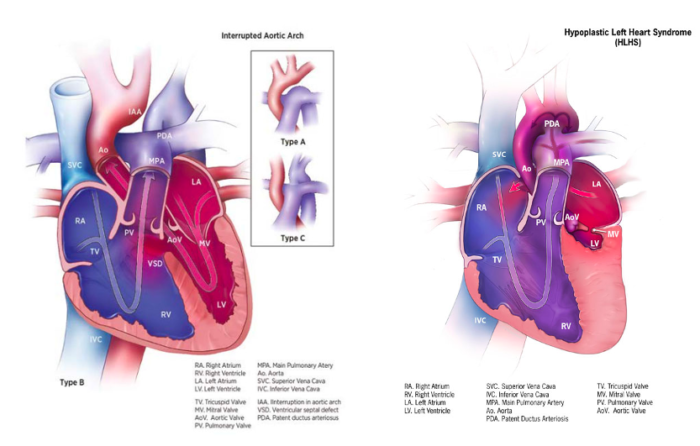
Figure 3. Ductal-dependent systemic circulation: Interrupted aortic arch and hypoplastic left heart syndrome. Source: Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities. Public Domain.
Lesions with Ductal-Dependent Mixing
- It is caused by parallel circulations with inadequate mixing, such as d-transposition of great arteries (d-TGA). Please see the OA summary on d-TGA for more details. Link
- The DA provides intercirculatory mixing and/or indirectly improves mixing at the atrial level, resulting in a higher proportion of effective blood flow through parallel pulmonary and systemic circulation.
- Inadequate intercirculatory mixing leads to insufficient tissue oxygen delivery and results in worsening metabolic acidosis and hypercarbia.
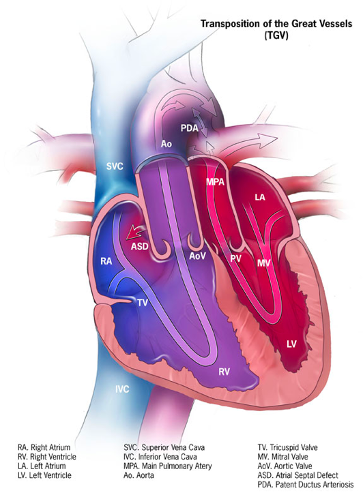
Figure 4. Ductal-dependent mixing: d-TGA. In infants with d-TGA and intact ventricular septum, intercirculatory mixing occurs at two main sites: PFO and DA. In the presence of a nonrestrictive atrial communication (PFO) and low PVR, the DA shunts deoxygenated blood from the aorta to the PA, where it gets oxygenated in the lungs, then crosses the atrial septum (PFO), and provides a portion of oxygenated blood to the systemic circulation. Source: Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities. Public Domain.
Associated Anomalies
- Extracardiac anomalies in neonates with ductal-dependent CHD are not uncommon and vary widely based on the lesion.
- Associated syndromes, such as trisomy 21, chromosome 22Q11.2 deletion, and VACTERL syndrome, are commonly encountered by pediatric anesthesiologists.
- It is imperative to maintain ductal patency during emergent noncardiac surgeries, such as tracheoesophageal fistula, duodenal atresia, or imperforate anus repair.
Treatment Strategies
Medical Management (PGE1)
- PGE1 is a naturally occurring prostaglandin that is FDA-approved for use in ductal-dependent CHD until palliative or corrective surgery can be performed.1
- The indications to commence PGE1 infusion are broadly divided into 3 categories:
- antenatal diagnosis of duct-dependent CHD
- neonatal cyanosis not responsive to O2 in the absence of pulmonary pathology
- neonate with weak/absent femoral pulses and evidence of systemic hypoperfusion without alternative etiology of shock (e.g., sepsis, metabolic disease)
- The exact mechanism of PGE1 vasodilation specific to the DA is not well delineated. However, PGE1 appears to result in dose-related increase in nitric oxide and nitric oxide synthase in other tissues.
- The ductus usually reopens within 30 minutes to 2 hours of initiating PGE1. The clinical response is usually instant if the ductus is vital for maintaining hemodynamic or oxygenation status.2
- Reversing ductal closure with PGE1 treatment is more likely to be successful before the ductus becomes permanently closed.
- The usual starting dose is 0.05 mcg/kg/min, which can be doubled every 15-30 minutes up to 0.2 mcg/kg/min until a clinic response is achieved. Once the ductus is open, patency can usually be maintained with low doses of 0.01 to 0.025 mcg/kg/min.3
- PGE1 is rapidly metabolized in neonates with 80% breaking down on a single pass through the lungs. As a result, PGE1 requires a continuous intravenous infusion.
- The common adverse effects associated with PGE1 therapy are hypotension, peripheral vasodilation, fever, and respiratory depression, including apnea. This occurs in 12% of children and is most common in infants with a birth weight < 2 kg.2,4 In patients on long-term treatment with PGE1, rare complications such as cortical hyperostosis and intimal mucosal damage have been reported.2,4,5
Interventional Management
Palliation
- Stenting of the DA may be performed in the cardiac catheterization laboratory.
- This strategy is particularly useful in high-risk preterm neonates.
- Advantages over surgical palliation include avoidance of sternotomy and/or cardiopulmonary bypass and a decrease in postoperative complications (e.g., thrombosis, shunt occlusion, and end-organ hemodynamic instability).
Repair
- Obstructive right- and left-sided valvar lesions may be corrected with balloon valvotomy or dilation.
Surgical Management
- Ductal-dependent pulmonary circulations are generally managed with palliative systemic-to-pulmonary shunts.
- Ductal-dependent systemic circulations are generally either managed with palliative stabilization (e.g., Damus-Kaye-Stansel procedure with arch reconstruction in HLHS) or definitive surgical repair (e.g., coarctation or interrupted aortic arch repair).
Anesthesia Considerations
Overall Goals
- Patients with ductal-dependent CHD should be cared for in tertiary medical centers with appropriate anesthesia, critical care, interventional cardiology, and surgical capabilities.
- The primary goal is the prevention of end-organ injury. In patients with ductal-dependent pulmonary and systemic circulations, systemic oxygen delivery and perfusion are optimized by balancing the systemic and pulmonary circulations (QP:QS ratio).
- QP:QS > 1:1 is associated with a decrease in systemic oxygen delivery due to a decrease in systemic blood flow.
- QP:QS < 1:1 is associated with a decrease in systemic oxygen delivery due to a decrease in systemic oxygen content.
Ventilation
- Excessive Qp should be avoided by titrating ventilation to maintain mild hypercarbia (PaCO2 45-55mmHg), PaO2 of 40-45mmHg, and SaO2 of 80-85%.
- High FiO2 should be avoided.
- Functional residual capacity should be maintained (tidal volume 8-12 mL/kg, 2-5cm H20 of PEEP) to avoid atelectasis and intrapulmonary shunting.
Hemodynamic Support
- A prostaglandin infusion should be continued via a dedicated line until a definitive source of pulmonary (or systemic) blood flow is established.
- Inotropic support with dopamine, epinephrine, or norepinephrine may be needed to support cardiac output and maintain adequate diastolic pressures in the setting of an unrestricted patent DA. Vasoactive support with phenylephrine or vasopressin may be necessary in some cases.
- Systemic vasodilator therapy, such as milrinone, decreases SVR and may help to promote Qs and improve DO2.
- Oxygen delivery is highly dependent on hemoglobin concentration. A hematocrit of ~40% should be targeted in cyanotic lesions.
- Adequate end-organ perfusion should be monitored by measuring base deficit, lactate, mixed venous oxygen saturation (if possible), urine output, and tissue oximetry.
References
- Roehl SL, Townsend RJ. Alprostadil (Prostin VR Pediatric Sterile Solution, The Upjohn Company). Drug Intell Clin Pharm. 1982;16(11):823-32. PubMed
- Freed MD, Heymann MA, Lewis AB, et al. Prostaglandin E1 infants with ductus arteriosus-dependent congenital heart disease. Circulation. 1981;64(5):899-905. PubMed
- Sarah Tabbutt MAH, and David G. Nichols. Critical Heart Disease in Infants and Children. Second ed. Philadelphia: MOSBY-ELSEVIER; 2006. 196 p.
- Lewis AB, Freed MD, Heymann MA, et al. Side effects of therapy with prostaglandin E1 in infants with critical congenital heart disease. Circulation. 1981;64(5):893-8. PubMed
- Akkinapally S, Hundalani SG, Kulkarni M, et al. Prostaglandin E1 for maintaining ductal patency in neonates with ductal-dependent cardiac lesions. Cochrane Database Syst Rev. 2018;2(2):CD011417. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.