Copy link
Pharmacogenetics of Analgesics
Last updated: 07/10/2025
Key Points
- The variance in clinical response and side effect profiles of opioids can be explained by pharmacogenetics.
- Genetic variants of metabolizers, transporters, and receptors significantly contribute to the observed variance, while notable developmental changes also play a role.
- Understanding these differences and their outcomes may lead to improved and personalized care in the future.
- The future of pain management involves personalized, precision-based interventions, guided by genomics and supported by real-time clinical tools.
Basic Concepts, Background, and Nomenclature
- Interindividual variability in pain perception and genetic differences in drug metabolism and response have resulted in up to 40% of patients experiencing inadequately controlled pain postoperatively and serious side effects, including respiratory depression and death.1,2 Besides clinical impact, opioid-related adverse drug events are associated with a 55% increase in length of hospital stay, 47% higher cost, and significantly increased risk of mortality.3
- Twin studies have found significant heritability for opioid-induced respiratory depression (30%), nausea (59%), drug disliking (36%), sedation (29%), pruritus (38%), dizziness (32%), and drug liking (26%).4 This further suggests the involvement of genetics in interindividual variability of analgesia.
- The term “pharmacogenomics” was coined in the 1990s, coinciding with the establishment of the Human Genome Project and the emergence of the discipline of genome sciences. The term is an acknowledgement of the term “pharmacogenetics,” which was defined by Friedrich Vogel in 1959 as the role of genetic variation in affecting drug response.
- Advances in genetic sciences have enabled not only the identification of the entire human genome but also the discrimination of more specific gene variants, known as single-nucleotide polymorphisms (SNPs), which occur when a single base substitution in a DNA sequence occurs at a significant rate in a population.5
- Nomenclature: There are several ways to name variants, including: base changes and location, dbSNP ID, changes at the level of DNA or protein, or by use of star alleles (see examples in Table 1). Since DNA is double-stranded, variants may be homozygous or heterozygous depending on whether the nucleotide change happens on both or one strand, respectively. Another way to delineate variants is by diplotype or copy number variant and its associated function (see CYP2D6 example in Table 1).

Table 1. Nomenclature examples of gene variants
- Variants can impact gene function and both drug metabolism → exposure → effect. Table 1 shows an example of classes of gene variants that impact both pharmacokinetics and pharmacodynamics of opioids.
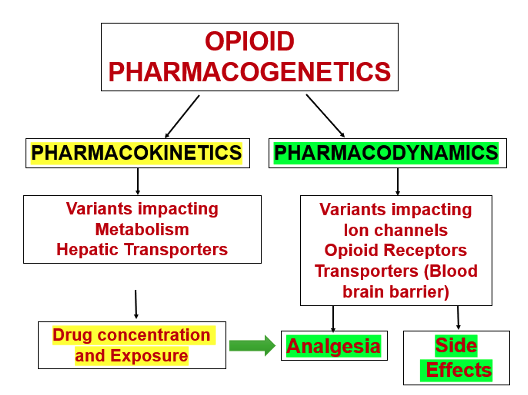
Figure 1. Basic concepts to understand how pharmacogenetics influences opioid effects
Hepatic Metabolism: Genetic Impact on Opioid Pharmacokinetics
Phase I Reactions
- Phase I reactions are the initial steps in drug metabolism that add polar groups through oxidation, reduction, and hydrolysis, making downstream metabolites more water-soluble. They are primarily carried out by CYP450 1, 2, and 3 families.
- CYP450 genes are of particular importance to pain management as their isoforms are involved in the metabolism of many of the analgesic agents. Table 2 lists commonly used perioperative analgesics and the corresponding cytochrome P450 (CYP) enzymes involved in their metabolism.1
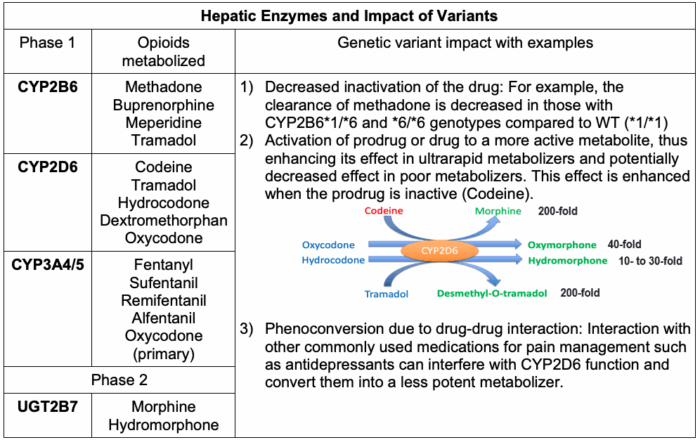
Table 2. Hepatic enzymes and impact of variants
-
- CYP3A4 metabolizes over 50% of all drugs, meaning opioids metabolized by this enzyme have a high risk of drug-drug interactions.
- CYP2D6 variations and impact: There are over 100 known functional and nonfunctional polymorphisms of CYP2D6, but of particular interest are those that contribute to gain or loss of function.
- CYP2D6*# is the convention used to distinguish various alleles in the CYP2D6 family (Table 1). CYP2D6*1 is the wild-type allele with normal enzymatic function; known variants *2, *33, and *35 also have near-normal enzymatic function.
- The polymorphisms found in CYP2D6*3, 4, 5, 6, and 9 result in a loss of enzymatic function. These phenotypes are referred to as poor metabolizers and will have no clinical response to codeine as they are unable to convert the prodrug to morphine. About 90% of poor metabolizers have one of these polymorphisms.6
- CYP2D6*10,17,41 are polymorphisms that produce the phenotype of an intermediate metabolizer that have somewhat reduced enzymatic function and variable clinical response.7
- When an individual has multiple functional alleles, this confers the phenotype of ultra-metabolizer. If these patients are administered appropriate doses of codeine, large amounts of morphine may be produced rapidly, leading to side effects or, in some pediatric cases, accidental death.
- The United States Food and Drug Administration released a safety announcement stating that they are restricting the use of codeine and tramadol in children, and they recommend against the use of codeine and tramadol in breastfeeding mothers due to possible harm to their infants.1,6 Link
Phase II Reactions
- Phase II reactions further increase the hydrophilicity through conjugation of downstream drug metabolites to prepare them for renal elimination.1,5
- Conjugation reactions include glucuronidation and sulfation.
- Glucuronidation is carried out by uridine diphosphoglucuronosyltransferases (UGTs).1
- Generally, UGTs reach mature adult levels of activity by 2 years of age.
- In contrast to UGTs, sulfotransferase enzymes display fully mature levels of activity at birth and may compensate for the less active UGTs.1
Hepatic Transporters
- Organic cation transporter 1 (OCT1, encoded by SLC22A1) is highly expressed in the basolateral membrane of hepatocytes. It mediates the hepatic uptake of cationic drugs, including morphine.
- OCT1 loss of function variants lead to reduced hepatic uptake of morphine, resulting in higher plasma concentrations and prolonged analgesic effect or increased risk of side effects.
- ATP-binding cassette subfamily c member 3 (ABCC3, also known as MRP3) is located on the basolateral side of hepatocytes and functions as an efflux transporter, moving opioid glucuronide metabolites (e.g., morphine-3-glucuronide, morphine-6-glucuronide) into the bloodstream.
- Enhanced ABCC3 activity due to variants may lead to higher systemic levels of active glucuronide metabolites, particularly morphine-6-glucuronide.
Developmental Pharmacogenomics
- Age-related differences in response to medications can be attributed to the immaturity of the liver and its metabolic enzymes at birth, and are of particular importance in the field of pediatrics.5
- The metabolism of opioids changes as the liver develops in utero, matures through infancy, and finally reaches adult levels of enzymatic competency. The age at which full maturation is realized varies depending on the given enzyme.
- The transition from fetal to neonatal circulation induces significant changes in blood flow, particularly to the liver.
- Intrauterine substance exposure may induce certain CYPs, leading to unpredictable patterns of drug effects and side effects.1
- Substantial genetic variation and developmental changes confer a large interindividual variability in response to a given medication.1
- Table 3 below summarizes the developmental changes of just two of the main CYPs involved in opioid metabolism.
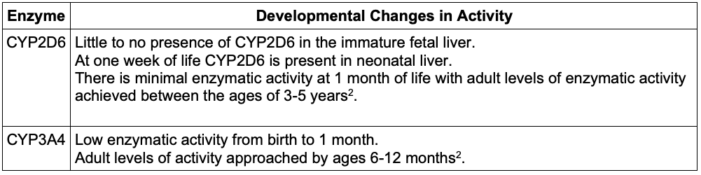
Table 3. Developmental changes of the main CYPs involved in opioid metabolism
Genomics of Pain Perception and Opioid Pharmacodynamics
- Pain perception and genetics
- Pain is a subjective response to a stimulus that is shaped by genetic, psychological, and environmental factors.
- Catechol-O-methyltransferase (COMT) regulates the breakdown of catecholamines (dopamine and norepinephrine) that modulate pain perception.1
- There are four well-known SNPs (rs6269, rs4633, rs4818, and rs4680) found in the COMT gene that contribute to low, average, and high pain sensitivity phenotypes.1
- Another enzyme involved in the synthesis of catecholamines and nitric oxide is GTP cyclohydrolase 1 (GCH1). GCH1 is the rate-limiting enzyme involved in the production of an essential cofactor in tetrahydrobiopterin (BH4).
- Reduced pain sensitivity has been noted in patients with loss-of-function GCH1 haplotypes.1
- These are just two examples of genes that contribute to the perception of pain and may predict opioid consumption as it pertains to pain sensitivity. In the future, multigene models may provide valuable clinical information for individualized opioid needs.1
- Genetic Variations Affecting Opioid Pharmacodynamics
- Figure 2 shows various transporters and receptors involved in opioid uptake1:

Figure 2. Morphine and codeine metabolism and transport Adapted from Chidambaran V, et al. Opioid-induced respiratory depression: the role of genetics. Expert Review of Precision Medicine and Drug Development. 2017;2(3):157-168.1
-
- The μ-opioid receptor gene (OPRM1) has been extensively studied.
- One well-known SNP (SNP A118G) causes the loss of an N-glycosylation site on the extracellular portion of the receptor, altering ligand-receptor binding1 and thereby diminishing the clinical response to opioids.
- This SNP is more prevalent in Asian people (50%) with a lower prevalence in Caucasian people (10.5-18.8%) and African American people (5-15%).
- The blood-brain-barrier transporter or p-glycoprotein efflux transporter (ABCB1) is known to transport morphine, fentanyl, methadone, sufentanil, and alfentanil.
- SNPs of this transport protein have been linked to respiratory depression and opioid associated nausea and vomiting.
Pharmacogenomics of Nonopioid Analgesics
- Nonsteroidal anti-inflammatory drugs (NSAIDs)
- NSAIDs like ibuprofen are initially broken down by CYP2C9 and then undergo further glucuronidation by UGT2B7.
- In vivo and in vitro studies have demonstrated that CYP2C9*1, 2, and 3 reduce the metabolism of NSAIDs.5
- Acetaminophen
- Acetaminophen (APAP) undergoes glucuronidation (52-57%) and sulfonation (30-44%)1, and a minority of the substrate is converted to N-acetyl-p-benzoquinone imine (NAPQI).1
- Glucuronides are formed by UGTs encoded by UGT1A1, UGT1A6, UGT1A9, and UGT2B15.
- Sulfonation is accomplished by SULTs encoded by SULT1A1, SULT1A3
- NAPQI is formed primarily by CYP2E1.1
- When toxic doses of acetaminophen are ingested, the metabolic pathways become saturated, resulting in high levels of NAPQI that deplete glutathione stores, eventually resulting in hepatic necrosis.1
- In vitro studies have shown that CYP2E1 activity levels are significantly lower in infants younger than 3 months.1
Genetics of Chronic Postsurgical Pain
- Genes, environment, and pain susceptibility
- There is a significant genetic contribution to acute and chronic postsurgical pain (CPSP) susceptibility.8 Systematic reviews have identified only a handful of gene variants predictive of CPSP, largely due to the heterogeneity of outcome definitions and surgical populations.9
- Patients prone to developing persistent postoperative pain demonstrate altered genetic expression in genes involved in the entirety of the pain pathways from peripheral nociception (TRPA1/TRPV1) to the dorsal horn (brain-derived neurotrophic factor, BDNF).10
- Proinflammatory states, characterized by the upregulation of interleukins (IL-6) and tumor necrosis factor, are associated with alterations in pain pathways and their respective receptors.
- Polygenic risk scores (the sum of the products of the risk conferred by several variants) have been proposed in surgical pediatric patients, showing high predictive accuracy for CPSP.
Clinical Translation: Moving from Bench to Bedside
- Emerging omics sciences—transcriptomics, proteomics, metabolomics, and epigenetics—add to the understanding of mechanisms underlying pain chronification and variability in drug response. For example, genetic variants and epigenetic changes in the opioid receptor gene OPRM1 have been studied in relation to postsurgical pain.
- With decreasing costs of genetic tests, precision pain management informed by molecular profiling has made its way to in-hospital and clinic use, for example, in chronic pain clinics and management of patients with sickle cell disease. This helps minimize the trial-and-error approach to treatment. The integration of pharmacogenomic testing into electronic health records enables clinicians to tailor analgesic therapy based on individual genetic profiles. Decision-support tools integrated into clinical workflows (e.g., Epic, Cerner) provide dosing recommendations, alerts for drug-gene interactions, and highlight alternatives when pharmacogenomic risk is identified.
- Several commercial pharmacogenomic panels are now available to guide analgesic prescribing. Some examples are listed below:
- GeneSight® Psychotropic (Myriad Genetics) – includes CYP2D6 and CYP2C19, with implications for tramadol and other drugs.
- OneOme® RightMed – comprehensive panel including CYP2D6, CYP3A4/5, OPRM1, COMT, and others.
- Admera Health PGxOne™ Plus – evaluates 50+ genes, including pain-relevant pathways.
- Genomind Professional PGx Express™ – includes pain, psych, and CNS-targeted gene-drug interactions.
Conclusions
- While current pain treatments often follow a one-size-fits-all model, pharmacogenomics offers a data-driven alternative.
- By harnessing genetic information to individualize analgesic strategies, clinicians can improve efficacy, reduce adverse events, and contribute to safer opioid prescribing.
- The future of pain management involves personalized, precision-based interventions, guided by genomics and supported by real-time clinical tools.
References
- Chidambaran V, McAuliffe JJ. Opioid-induced respiratory depression: the role of genetics. Expert Review of Precision Medicine and Drug Development. 2017;2(3):157-168. Link
- Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149-60. PubMed
- Oderda GM, Said Q, Evans RS, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41(3):400-6. PubMed
- Angst MS, Phillips NG, Drover DR, et al. Pain sensitivity and opioid analgesia: a pharmacogenomic twin study. Pain. 2012;153(7):1397-409. PubMed
- Packiasabapathy S; Chidambaran V; Sadhasivam S. Pharmacogenomics. In: Coté CJ, Lerman J, Anderson B. A Practice of Anesthesia for Infants and Children. 6th Edition. Philadelphia, PA; Elsevier; 2019: 81-99.
- Chidambaran V, Sadhasivam S, Mahmoud M. Codeine and opioid metabolism: implications and alternatives for pediatric pain management. Curr Opin Anaesthesiol. 2017; 30(3):349-56. PubMed
- Matic M, Nijenhuis M, Soree B, et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene–drug interaction between CYP2D6 and opioids (codeine, tramadol and oxycodone). European Journal of Human Genetics. 2021; 30(10):1105-13. PubMed
- Chidambaran V, Ashton M, Martin LJ, Jegga AG. Systems biology-based approaches to summarize and identify novel genes and pathways associated with acute and chronic postsurgical pain. J Clin Anesth. 2020;62:109738. PubMed
- Chidambaran V, Gang Y, Pilipenko V, Ashton M, Ding L. Systematic Review and Meta-Analysis of Genetic Risk of Developing Chronic Postsurgical Pain. J Pain. 2020;21(1-2):2-24. PubMed
- Dourson AJ, Willits A, Raut NGR, et al. Genetic and epigenetic mechanisms influencing acute to chronic postsurgical pain transitions in pediatrics: Preclinical to clinical evidence. Can J Pain. 2022;6(2):85-107. PubMed
Other References
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.