Copy link
Congenital Heart Diseases and Pregnancy: Part 2
Last updated: 10/02/2025
Key Points
- Proper counseling of parturients with congenital heart disease (CHD) prior to conception is critical.
- For certain conditions, pregnant patients should be cared for at tertiary care centers with the highest level of critical care access (including mechanical circulatory support).
- Early interdepartmental communication is important for a successful outcome.
- Whenever possible, delivery plans should be discussed ahead of time, and the obstetric and cardiac anesthesiology teams should be involved early.
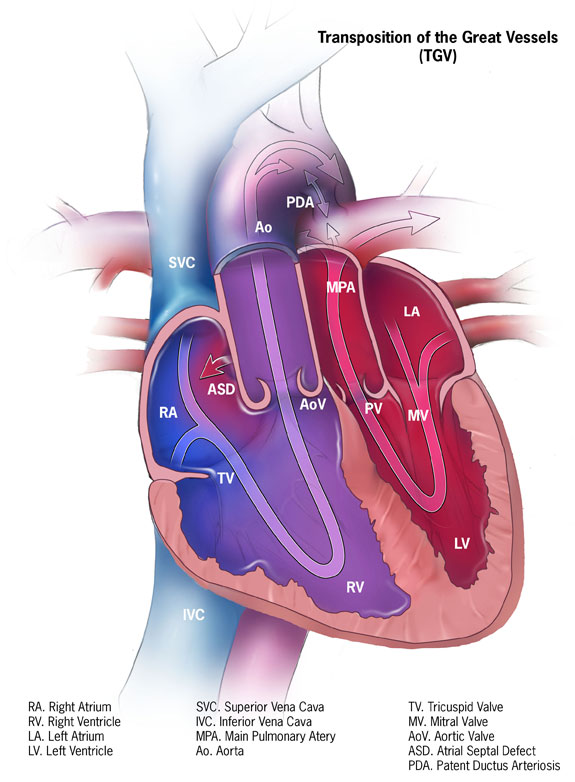
Dextro-Transposition of Great Arteries
Background Pathophysiology
- Dextro-transposition of the great arteries is a congenital heart defect in which the aorta and pulmonary artery are switched in position, creating two separate circulatory systems with no mixing.
- The aorta exits the left ventricle (LV), and the pulmonary artery exits the right ventricle (RV).
- Complete transposition requires surgical intervention within the first few weeks of life and presents with cyanosis in the neonate.
- The most common surgical procedure is now the arterial switch procedure, in which the aorta and pulmonary artery are transected and anastomosed to their correct ventricles.1
- The atrial switch procedure involves creating a baffle within the atria to redirect blood flow. The RV functions for the systemic circulation, and the LV for the pulmonary circulation.2
Maternal and Fetal Considerations
- There is an elevated risk of maternal arrhythmia.1
- If the parturient had an atrial switch procedure, they are more likely to have RV failure as the RV is functioning as the systemic ventricle, working against a higher systemic vascular resistance (SVR).2
Anesthetic Considerations
- A transthoracic echocardiogram (TTE) should be considered in parturients with surgically corrected transposition of great arteries (TGA).1
- Parturients after arterial switch procedures generally tolerate pregnancy well.2
- Both neuraxial and general anesthesia can be considered acceptable.1
- Caution is advised with excessive preload due to the risk of RV strain or failure, and central venous pressure monitoring may be necessary.1,2
- Continuous electrocardiogram (ECG) monitoring during labor and delivery is strongly recommended due to the high risk of arrhythmias. This requires antepartum planning, and depending on the hospital setting and available staff, labor and delivery may need to take place in an intensive care unit (ICU).1
Levo-TGA1,2
Background Pathophysiology
- L-TGA occurs when the aorta and pulmonary artery are reversed in their origins from the heart, and the ventricles are also inverted, such that the morphologic LV is on the right.
Maternal and Fetal Considerations
- Pregnancy is often tolerated well.1,2
Anesthetic Considerations
- Parturients should undergo TTE to assess ventricular function.2
- Both neuraxial and general anesthesia can be considered acceptable.1,2
Ebstein’s Anomaly1
Background Pathophysiology
- Occurs when the tricuspid valve is positioned abnormally with larger valve leaflets that are displaced downward in the ventricle toward the apex.
- This can often cause significant tricuspid regurgitation and an enlarged RV.
Maternal and Fetal Considerations
- Arrhythmias, such as atrial fibrillation, flutter, and tachycardia, are common.1
- If the parturient has normal ventricular function, pregnancy is often well tolerated.1
Anesthetic Considerations
- Continuous ECG monitoring should be considered due to the high incidence of arrhythmias.1
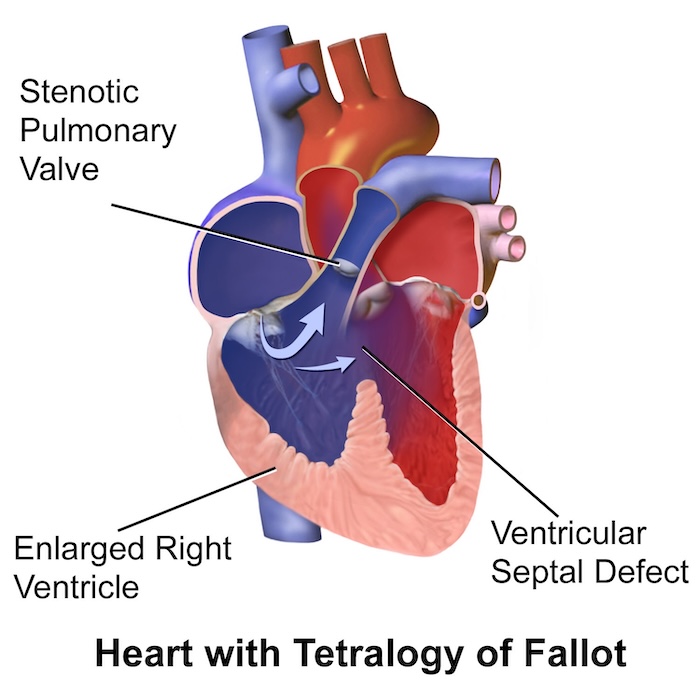
Tetralogy of Fallot1,3,4
Background Pathophysiology
- Tetralogy of Fallot (TOF) is a rare congenital heart defect involving four lesions: ventricular septal defect (VSD), overriding aorta, pulmonary stenosis, and right ventricular hypertrophy.
- Often diagnosed in utero or shortly after birth, but depending on the severity of the disease, it might not be diagnosed until adulthood.
- The standard treatment for TOF is definitive surgical repair.
- Often, patients with unrepaired TOF experience “tet spells” characterized by cyanosis caused by decreased oxygenation or sudden drops in SVR, which lead to increased right-to-left shunting through the VSD.
Maternal and Fetal Considerations
- The strain of pregnancy on the heart, particularly for parturients with unrepaired TOF, can lead to serious health issues, including right ventricular dilation and failure and arrhythmias.1
- Patients with repaired TOF generally tolerate pregnancy well; however, those with unrepaired TOF, especially if they remain cyanotic, are considered high risk and pregnancy is not advised.1,3
Anesthetic Considerations
- With unrepaired TOF, careful attention should be paid to ensure adequate SVR and intravascular volume to prevent right-to-left shunting and cyanosis.1,3
- Case reports have demonstrated successful delivery both vaginally and with cesarean section.4,5
- For a cesarean section, a titratable neuraxial anesthetic, such as a slowly administered epidural, can be considered.1,3
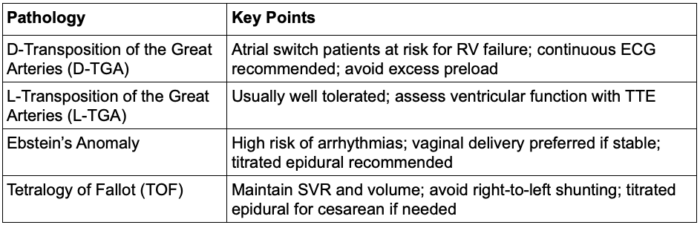
Table 1. Congenital heart defects: part 2 summary. Abbreviations: ECG, electrocardiogram; TTE, transthoracic echocardiogram
Formulating Delivery and Anesthesia Plans for Complex Obstetric Patients
- In general, it is best to consider these patients as a consult before they arrive at the hospital.
- Planning early in a multidisciplinary team (Maternal Fetal Medicine, Obstetric Anesthesiology, Cardiology, etc.) is essential.
- Delivery mode – vaginal vs. cesarean delivery. If the patient labors, consider assisted delivery during the second stage to minimize Valsalva.
- Intrapartum monitoring – if continuous telemetry is needed, arrange for a telemetry nurse to be present throughout labor, depending on the hospital setting. Sometimes, this requires delivering in the ICU. A separate anesthesiology team may need to manage these patients.
- Early hospital admission is advised to ensure complex plans can be carried out.
- Postpartum monitoring – many of these patients are at a higher risk for arrhythmias after delivery due to autotransfusion. These patients may need to go to the ICU following delivery for closer observation.
References
- Chestnut D, Wong C, Tsen L, et al. Chestnut’s Obstetric Anesthesia: Principles and Practice. 6th Edition, April 2019.
- Canobbio MM, Warnes CA, Aboulhosn J, et al. Management of pregnancy in patients with complex congenital heart disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135(8):e50–e87. PubMed
- Garagiola ML et al. Pregnancy considerations in tetralogy of Fallot. CJC Pediatr Congenit Heart Dis. 2023;2(6Part A): 301-13. PubMed
- Veldtman GR et al. Outcomes of pregnancy in women with tetralogy of Fallot. J Am Coll Cardiol. 2004;44(1):174-80 PubMed
- Arendt KW, Fernandes SM, Khairy P, et al. A case series of the anesthetic management of parturients with surgically repaired tetralogy of Fallot. Anesth Analg. 2011;113: 307–17. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.