Copy link
Milrinone
Last updated: 10/28/2025
Key Points
- Milrinone is a phosphodiesterase-3 (PDE3) inhibitor used in the short-term management of acute decompensated heart failure, right ventricular (RV) dysfunction, pulmonary hypertension, and perioperative low cardiac output states.
- It enhances cardiac contractility, improves diastolic relaxation, and produces systemic and pulmonary vasodilation by increasing intracellular cyclic adenosine monophosphate (cAMP), maintaining efficacy even in patients on chronic β-blocker therapy.
- Adverse effects include dose-dependent hypotension and ventricular arrhythmias, with increased risk in patients with renal dysfunction due to its primary renal clearance.
Physiochemical Characteristics
- Milrinone is a bipyridine methyl carbonitrile compound, making it chemically unrelated to catecholamines.1
- Milrinone has an acidic pH range (~3.0–4.0), requiring cautious compatibility with other intravenous (IV) medications.
- Milrinone does not require reconstitution and is stable in D5W and NS for limited time periods.
Mechanism of Action
- Milrinone inhibits PDE3, preventing cAMP breakdown in cardiac and vascular smooth muscle. It acts downstream of the β1 receptor, retaining efficacy even in patients with β-blockade or chronic receptor desensitization.1,2
- Milrinone reduces preload and afterload without a significant chronotropic effect by increasing cAMP in cardiac myocytes, which enhances intracellular Ca²⁺ (resulting in increased inotropy), and in vascular smooth muscle, where elevated cAMP causes vasodilation.3
- Milrinone improves diastolic relaxation (increased lusitropy) by activating protein kinase A to enhance Ca²⁺ reuptake into the sarcoplasmic reticulum via SERCA2a.4
- Compared to catecholamines, milrinone improves cardiac output with a lower rise in myocardial oxygen demand.5
Pharmacokinetics
- The pharmacokinetics of milrinone are listed in Table 1.
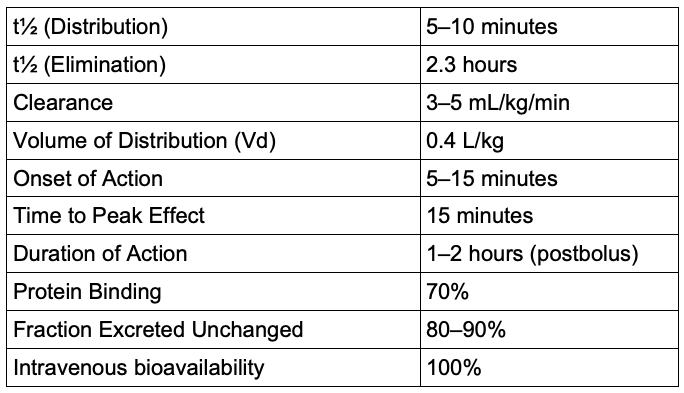
Table 1. Pharmacokinetics of milrinone
- Milrinone is administered either IV (most common) or inhaled via nebulization. There is no oral formulation due to poor GI absorption and high first-pass metabolism.
IV Milrinone
- The onset of action begins within 5–15 minutes of IV loading. Distribution half-life is ~5–10 minutes.
Inhaled Milrinone
- Inhaled milrinone is rapidly absorbed through the lungs, reaching peak plasma levels within 10–30 minutes at the end of inhalation, with lower systemic exposure than IV administration.7
- It provides selective pulmonary vasodilation with minimal systemic hypotension. The effects wear off within 60 minutes.7
Metabolism and Elimination
- Milrinone undergoes little to no metabolism in the liver.
- Instead, 80–90% is excreted unchanged in the urine. Clearance is directly proportional to renal function.
- With normal renal function, the elimination half-life is around 2–2.5 hours.
- In neonates and infants, immature renal function leads to markedly reduced clearance and prolonged elimination half-life (up to 6–8 hours). In contrast, in elderly adults, age-related decline in glomerular filtration rate also slows clearance.
Systemic Effects1
Cardiovascular
- Milrinone increases myocardial contractility (positive inotropy) and enhances diastolic relaxation by increasing intracellular cAMP and calcium in cardiac myocytes.
- Milrinone causes arterial and venous vasodilation, reducing both systemic and PVR. This causes a decrease in left and RV filling pressures.
- These effects improve cardiac output and reduce preload and afterload. It has minimal chronotropic effect.
- No significant increase in myocardial oxygen consumption has been observed at therapeutic doses.
Pulmonary
- Pulmonary vasodilation reduces pulmonary vascular resistance (PVR).
- Milrinone can enhance pulmonary oxygenation by increasing RV output and pulmonary blood flow, achieved through reduced RV afterload and improved RV contractility.8
Renal
- Milrinone increases renal blood flow secondary to improved cardiac output and vasodilation.
Endocrine/Metabolic
- Milrinone can cause hypokalemia, likely secondary to increased catecholamine activity and increased renal potassium loss.
Other Systems
- No direct effects on hepatic, gastrointestinal, or central nervous systems have been described in the medical literature at therapeutic doses.
Clinical Uses
RV Failure
- Decreased PVR causes decreased RV afterload, and increased forward flow.
- Milrinone is especially beneficial in pulmonary hypertension or after pulmonary thromboendarterectomy.8
- Often combined with vasopressin for inotropy and pulmonary vasodilation while maintaining systemic blood pressure (BP)/coronary perfusion.3
- Use cautiously because systemic vasodilation may decrease coronary perfusion if the BP is borderline.
Acute Decompensated Heart Failure
- Milrinone is often used in low-output heart failure, i.e., states when there is poor perfusion despite adequate volume and preload.
- It is preferred over dobutamine in patients with chronic β-blocker use or downregulated β-receptors.3
- Milrinone is not recommended as first-line therapy in hypotensive patients due to its vasodilatory properties.9
Use in Perioperative Setting9
- Coronary artery bypass graft: Used to increase cardiac output and reduce the need for high-dose catecholamines.
- Cardiac transplantation: Used to optimize graft function, reduce PVR, and facilitate weaning from cardiopulmonary bypass (CPB).
- Valve surgeries: Used where postoperative ventricular dysfunction or pulmonary hypertension is anticipated.
- Useful in weaning patients with preexisting left ventricular (LV) dysfunction from CPB.
- Applicable in select high-risk noncardiac surgeries with acute decompensated LV/RV failure.
Low Cardiac Output Syndrome After Cardiac Surgery
- Milrinone increases cardiac output without increasing heart rate or myocardial oxygen consumption.
- It is particularly helpful with β-blockade or β-receptor desensitization.3
- Avoid in vasoplegia or inadequate preload because of the risk of profound hypotension.9
Weaning from CPB8,9
- Milrinone is often initiated before separation from CPB to improve contractility and reduce afterload.
- It is often combined with vasopressors to maintain systemic perfusion.
- Compared to dobutamine, milrinone causes less tachycardia and better inotropic-lusitropic balance.
Pulmonary Hypertension7
- Milrinone is used to lower PVR and improve RV output, especially in RV failure or perioperative decompensation.
- It may be combined with vasopressors if hypotension is present.
- Compared to dobutamine: Decreases filling pressures, less tachycardia; preferred in β-blocked patients or when pulmonary vasodilation is desired
- The inhaled form minimizes systemic hypotension risk.
- Off-label: studied for persistent pulmonary hypertension of the newborn, especially in settings without inhaled nitric oxide/extracorporeal membrane oxygenation.
Special/Outpatient Use1
- Milrinone may be used as a bridge therapy for advanced heart failure patients awaiting transplantation or mechanical circulatory support
- It can also be used as a palliative option for patients with end-stage congestive heart failure, who are not eligible for advanced therapies
Adverse Effects1,6
- Hypotension: Due to both arterial and venous, especially after bolus dosing or in hypovolemic states. This can be exacerbated by having other vasodilators in circulation.
- Arrhythmias: As a result of increased intracellular cAMP and calcium, milrinone can cause premature ventricular contractions, supraventricular tachycardia, ventricular fibrillation, or ventricular tachycardia. Increased risk in patients with preexisting arrhythmias, metabolic abnormalities (especially hypokalemia), abnormal digoxin levels, or those with intracardiac catheters. Milrinone can also increase the ventricular response rate in patients with atrial flutter or fibrillation, especially if not adequately rate-controlled.
- Angina: This may result from milrinone’s positive inotropy and vasodilation (decreased coronary perfusion pressure), particularly in patients with coronary artery disease.
- Thrombocytopenia: Rare but documented. Less frequent than with amrinone. It is important to consider when the bleeding risk is already elevated.5
- Elevated liver enzymes: Mild transient increases in aspartate aminotransferase/alanine aminotransferase have been reported, but clinically significant hepatotoxicity is rare.
- Hypersensitivity reactions: Infusion site reactions, rash, bronchospasm, and rare cases of anaphylactic shock are possible with milrinone.
References
- Royster RL, Garner CR, Groban L, et al. Cardiovascular pharmacology. In: Hemmings HC, Egan TD, eds. Pharmacology and Physiology for Anesthesia: Foundations and Clinical Application. Vol 1. 2nd ed. Philadelphia, PA: Elsevier; 2019:221–44.
- Assaad SI, Heerdt PM, Crystal GJ. Cardiovascular physiology: cellular and molecular regulation. In: Hemmings HC, Egan TD, eds. Pharmacology and Physiology for Anesthesia: Foundations and Clinical Application. Vol 1. 2nd ed. Philadelphia, PA: Elsevier; 2019:455–84.
- Zimmerman J, Lee JP, Cahalan M. Vasopressors and inotropes. In: Hemmings HC, Egan TD, eds. Pharmacology and Physiology for Anesthesia: Foundations and Clinical Application. Vol 1. 2nd ed. Philadelphia, PA: Elsevier; 2019:519–544.
- Ayres JK, Maani CV. Milrinone. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. Accessed July 17, 2025. Link
- Levy JH, Bailey JM, Deeb GM. Intravenous milrinone in cardiac surgery. Ann Thorac Surg. 2002;73(1):325-30. PubMed
- U.S. National Library of Medicine. Milrinone Lactate Injection. DailyMed. Published 2018. Accessed July 15, 2025. Link
- Wang H, Gong M, Zhou B, Dai A. Comparison of inhaled and intravenous milrinone in patients with pulmonary hypertension undergoing mitral valve surgery. Adv Ther. 2009;26(4):462-8. PubMed
- Yamada T, Takeda J, Katori N, et al. Hemodynamic effects of milrinone during weaning from cardiopulmonary bypass: comparison of patients with a low and high prebypass cardiac index. J Cardiothorac Vasc Anesth. 2000;14(4):367-73. PubMed
- Nguyen AQ, Denault AY, Théoret Y, Varin F. Inhaled milrinone in cardiac surgical patients: pharmacokinetic and pharmacodynamic exploration. Sci Rep. 2023;13(1):3557. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.