Copy link
Cerebral Arteriovenous Malformations
Last updated: 03/14/2023
Key Points
- Cerebral arteriovenous malformations (AVM) may be congenital or de novo and may be asymptomatic or present with seizures, intracranial bleeding, or other neurological signs and symptoms.
- AVMs alter blood flow characteristics in the adjacent brain parenchyma causing chronic vasodilation and impairment in autoregulation.
- Strict hemodynamic control in the perioperative setting is essential to avoid complications such as intraoperative ischemia caused by hypotension and normal pressure breakthrough bleeding/edema caused by elevated blood pressures.
Definitions and Classifications
- Cerebral Arteriovenous malformations (AVMs) are abnormalities in which multiple direct arterial-to-venous connections exist without intervening capillaries. The pool of vessels is called the nidus, and they contain no neural tissue.
- Cerebral AVMs may be congenital or de novo malformations.
- AVMs are usually detected between the ages of 10 and 40 years, with approximately 70% being supratentorial. An aneurysm can be found on the feeding artery in 4-25% of AVMs.
- Other types of intracranial vascular malformations include venous angiomas, cavernous angiomas, capillary telangiectasias, and arteriovenous fistulas, which are outside of the scope of this summary.
- The Spetzler-Martin grading system is commonly used to predict surgical outcomes.1 It is based on the AVM’s size, eloquence of the adjacent brain, and pattern of venous drainage (superficial vs. deep) (Tables 1 and 2).
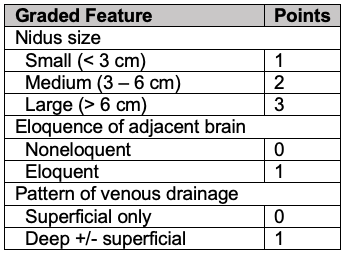
Table 1. Spetzler-Martin scoring of AVMs based on nidus size, eloquence, and pattern of venous drainage.1
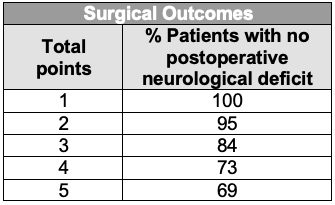
Table 2. Surgical outcome prediction based on Spetzler-Martin grade of AVMs.1
Pathophysiology2,3
- AVMs typically represent high-flow, low-resistance shunts, with intravascular pressure within the AVM being less than systemic arterial pressure.
- AVMs may lead to cerebral hemorrhage, headaches, seizures, cerebral edema, compression of the normal brain, vascular steal and/or new focal neurologic deficits.
- The cause of AVM-associated seizures is unclear but has been attributed to either steal (e.g., shunting of blood away from surrounding normal brain tissue toward the low-resistance AVM, causing ischemia) or gliosis due to hemosiderin deposits from previous hemorrhage.
- Rupture does not appear to be clinically associated with acute or chronic hypertension unless there is an accompanying aneurysm.
Intra- and Perioperative Blood Pressure Management3-5
- AVMs can be treated via an endovascular approach, surgical resection, radiosurgery, or using a combination of these modalities.
- Whether the risk of complications related to the natural history of AVMs (e.g., rupture, seizures etc.) outweighs the risks associated with various forms of treatment remains controversial and depends on multiple factors.2
- Large AVMs may require endovascular embolization a few days prior to surgical resection to decrease vascular flow and intraoperative blood loss and to allow for the surrounding brain parenchyma to adjust to the altered perfusion following embolization.
- During open surgical resection, strict control of blood pressure is required to maintain cerebral perfusion pressure (CPP) without worsening blood loss from the resection bed.
- At the same time, avoidance of hypotension is crucial as these patients often present with seizures or focal neurologic deficits due to ischemic “steal” phenomenon.
- After resection of AVM, maintenance of low normal blood pressure is imperative to prevent postoperative hyperemia and hemorrhage. The chronically vasodilated arterioles of brain adjacent to the resected AVM have lost their autoregulation capacity. These regions are susceptible to edema and hemorrhage even with normal blood pressure, a phenomenon called normal perfusion pressure breakthrough (NPPB).
- Avoidance of hypertension during emergence is imperative. Drugs such as β-adrenergic antagonists and calcium channel blockers (e.g. clevidipine, nicardipine) are often needed.
- Close hemodynamic and neurologic monitoring in the intensive care setting is necessary to reduce complications following AVM surgery.
Normal Perfusion Pressure Breakthrough Edema/Bleeding
- The phenomenon of NPPB is not fully understood, and two possible hypotheses have been proposed.4
- First, autoregulatory impairment caused by the AVM affects the surrounding brain tissue. The adjacent normal cerebral vessels have been maximally vasodilated due to longstanding “steal” caused by the AVM. When the AVM has been resected, these vessels are unable to vasoconstrict, leading to cerebral hyperemia, cerebral edema, headache, and possibly increased risk for postoperative bleeding (Figure 1).
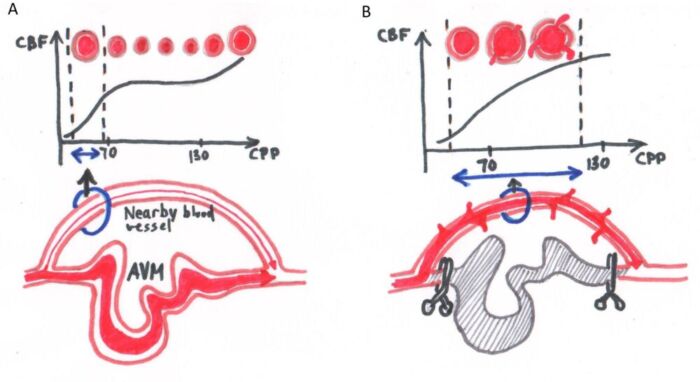
Figure 1. Normal perfusion pressure breakthrough. (A) Blood vessels near the AVM are chronically maximally dilated as a result of diminished blood flow because of shunting of blood by the AVM away from these vascular beds. (B) These blood vessels also lose their ability to autoregulate blood flow, and after exclusion of the AVM (e.g., surgical excision, embolization, etc.) the blood vessels fail to vasoconstrict in response to increased perfusion pressure and blood flow. Breakthrough edema and bleeding can occur even with normal perfusion pressures.
-
- Alternatively, “occlusive hyperemia” can also be considered. Stasis of blood and the development of microthrombi in the recently ligated feeder arterioles and draining veins can perturb local microcirculation in normal brain tissue that surround the AVM. This obstruction can impact flow, leading to ischemia from poor arterial flow and edema and an increased risk for hemorrhage from impaired venous outflow.
Implications for Anesthetic Management3,5
- Preoperatively, a patient with an intracranial vascular malformation should be evaluated for evidence of cerebral ischemia or increased intracranial pressure. The size, location, presence of associated aneurysms, and baseline neurological deficits should be known.
- Antiepileptic drugs should be continued through the perioperative period. Up to 50% of patients may experience seizures postoperatively, and prophylactic anticonvulsants are typically administered.
- For embolization or surgical resection of a vascular malformation in an eloquent region of the brain, anesthesia with intraoperative awakening and brain mapping (also known as “awake craniotomy”) is an attractive option for selected patients.
- Electrophysiological neuromonitoring is increasingly being used to facilitate cerebral AVM resections, and a maintenance anesthetic regimen that facilitates neuromonitoring is usually required.
- The greatest risk of AVM resection is bleeding. Intraoperatively surgical hemostasis can be challenging due to poor visualization of arterial and especially venous vessels. Postoperatively, the brain parenchyma around the resection is particularly prone to bleeding complications. Therefore, good IV access is of great importance, and blood products should be immediately available. Further, central venous access may be useful in some cases to monitor volume status and to allow rapid administration of large volumes of fluids, blood products, or vasoactive agents.
- Avoidance of significant blood pressure fluctuations during induction and intubation is paramount. An intraarterial catheter may be placed before induction of anesthesia.
- Techniques to blunt the hemodynamic responses to stimulating events, such as laryngoscopy, pinion placement, and skin incision, include ensuring an adequate depth of anesthetic with an optimal balance of hypnotic and analgesic agents. Careful titration of vasoactive medications may be required to reduce hypertensive episodes (e.g., esmolol, clevidipine) or hypotension (e.g., pressors and inotropes).
- In cases of large or high-flow vascular malformations, frequent communication with the surgeon is of paramount importance because of changing surgical requirements during various stages of resection of a large, complex lesion.
- Hypotonic and glucose-containing solutions should be avoided since the former can exacerbate cerebral edema, and the latter can worsen outcomes following neurologic ischemia.
• Administration of hyperosmolar medications (e.g., hypertonic saline or mannitol), mild hyperventilation (PaCO2 of 30–35 mm Hg), and maneuvers to decrease brain volume (e.g., drainage of cerebrospinal fluid, elevation of the head) help to facilitate surgical exposure. - An anesthetic technique that is conducive to rapid and controlled emergence from anesthesia at the end of surgery is always preferred for prompt neurologic assessment.
References
- Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65(4):476-83. PubMed
- Chen CJ, Ding D, Derdeyn CP, et al. Brain arteriovenous malformations: A review of natural history, pathobiology, and interventions. Neurology. 2020;95(20):917-27. PubMed
- Teig MK. Anesthetic management of patients undergoing intravascular treatment of cerebral aneurysms and arteriovenous malformations. Anesthesiol Clin. 2021;39(1):151-62. PubMed
- Rangel-Castilla L, Spetzler RF, Nakaji P. Normal perfusion pressure breakthrough theory: a reappraisal after 35 years. Neurosurg Rev. 2015;38(3):399-404; discussion 404-5. PubMed
- Miller C, Mirski M. Anesthesia considerations and intraoperative monitoring during surgery for arteriovenous malformations and dural arteriovenous fistulas. Neurosurg Clin N Am. 2012 ;23(1):153-64. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.