Copy link
Antifibrinolytics
Last updated: 12/12/2022
Key Points
- Antifibrinolytic agents are commonly used as part of a comprehensive blood management strategy to reduce bleeding, allogeneic blood administration, and transfusion-related adverse events.
- Despite their well-established role in various surgeries, the risk of seizures and theoretical concerns about their prothrombotic effects remain.
Fibrinolysis
Fibrinolysis is a protective physiologic process catalyzed by plasmin that allows fibrin clot breakdown into fibrin degradation products, thereby limiting clot size (Figure 1). This process removes excess fibrin deposition at the vascular injury site.1-3 After tissue and vascular injury, the coagulation pathway is activated, resulting in thrombin generation, platelet aggregation, cross-linking, and fibrin clot formation.2 Subsequently, endothelial activation by various agonists releases tissue plasminogen activator (tPA), which binds to the lysine residues on the fibrin clot. Once assembled within the clot, tPA cleaves plasminogen to its active form, plasmin, which breaks down the fibrin clot into fibrin degradation products. Plasmin can also be generated by urokinase, contact activation, and kallikrein-mediated protease activation.2
The physiological inhibitors of fibrinolysis includes the following: plasminogen activator inhibitor (PAI-1) which inhibits tPA, PAI-2 which inhibits urokinase-plasminogen activator, and direct plasmin inhibitors (⍺2-antiplasmin and ⍺2-macroglobulin).1,2 With an intact vascular and endothelial system, hemostatic balance is preserved.
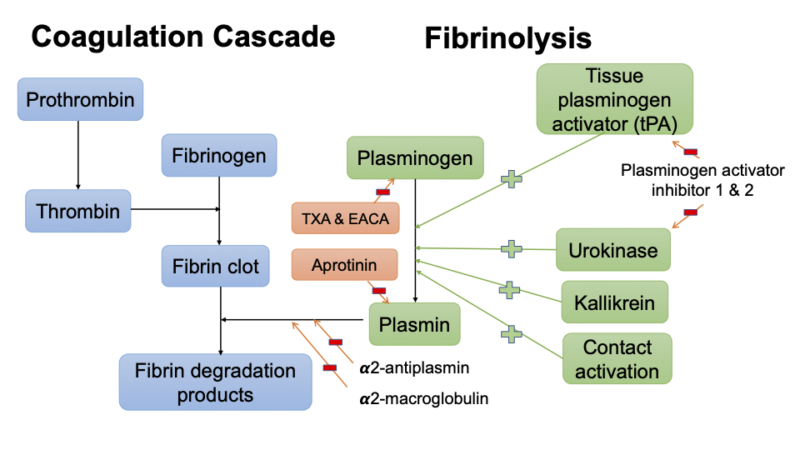
Figure 1. Simplified fibrinolysis pathway and site of action of anti-fibrinolytic agents.
After major trauma or surgery, ischemia/reperfusion, and extracorporeal circulation, excessive plasmin generation limits the ability to regulate fibrinolysis.2 Excess fibrinolysis has been identified as contributing factor to bleeding, coagulopathy, and inflammatory responses.2
Antifibrinolytic Agents
Antifibrinolytic agents are used to counteract excessive fibrinolysis and include tranexamic acid, epsilon aminocaproic acid, and aprotinin (Tables 1 and 2).
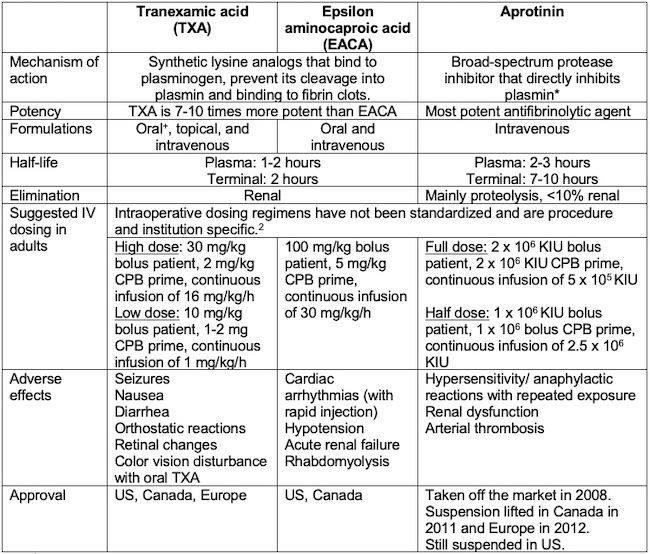
Table 1. *: Reversibly inhibits trypsin, kallikrein, plasmin elastase, urokinase & thrombin 1
+: Oral TXA approved for excessive menstrual bleeding.2
Table 1: Mechanism of action, pharmacokinetics, and side effects of antifibrinolytic agents (CPB = cardiopulmonary bypass, KIU = kallikrein-inhibiting units). Adapted from Koster A, et al. Anesthesiology. 2015;123(1): 214-21.3
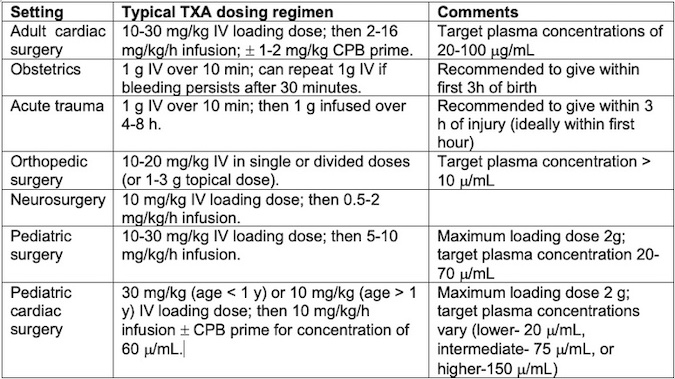
Table 2. Typical dosing regimens for perioperative TXA use. Adapted from Patel PA, et al. Anesth Analg. 2022;135(3): 460-73.4
Clinical Uses
Antifibrinolytics have an important role in blood conservation techniques in surgeries where major blood loss is expected. The benefit of transfusion reduction may be especially advantageous in situations where allogeneic transfusion is unacceptable or undesired (Jehovah’s witnesses, transplant candidates, etc.). Many randomized controlled trials (RCT) and meta-analyses support the efficacy of these agents in reducing blood loss and transfusion.1-5
Cardiac Surgery
- Surgical techniques that employ hypothermia and cardiopulmonary bypass circuits have an increased propensity to activate the fibrinolytic system. This allows antifibrinolytics to play a major role in reducing perioperative bleeding and allogeneic blood product transfusion.
- The use of both tranexamic acid (TXA) and epsilon aminocaproic acid (EACA) is well described in cardiac surgery, and their use has been shown to reduce postoperative bleeding in meta-analyses.1-5 The benefits are particularly important in high-risk cardiac surgeries such as redo sternotomies, multiple valve replacements, ascending aorta or aortic arch surgeries, or emergency procedures.3
- Retrospective data indicate that TXA may be slightly more effective at reducing postoperative bleeding, but more research is needed. Decisions regarding which lysine analog to use should be based on institutional availability and concern for side effects.1
Trauma Surgery
- About one-third of severely injured trauma patients are coagulopathic on hospital arrival, and the presence of acute traumatic coagulopathy increases patient mortality by more than 4-fold.1 Endothelial damage and tissue ischemia secondary to trauma activate the fibrinolytic system, which contributes to coagulopathy.
- The Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage-2 (CRASH-2) was a very large multinational, multicenter RCT that compared more than 20,000 adult trauma patients with significant bleeding who received TXA (1g bolus followed by 1 g infusion over 8 hours) to placebo.6 They found decreased all-cause mortality within 28 days and death due to bleeding in those receiving TXA. A follow-up analysis revealed that the benefit of TXA administration was greatest when administered within the first hour of trauma, and the mortality paradoxically increased when TXA was administered 3h after trauma. Since the CRASH-2 trial, the use of TXA in trauma patients has become common.
Orthopedic Surgery
- Tissue trauma and fluid shifts in major orthopedic surgery can lead to coagulopathy and hyperfibrinolysis.
- Antifibrinolytic use has been evaluated extensively in several orthopedic surgeries, including total hip and knee arthroplasty, spinal fusion, tumor surgery, etc.1,2 A meta-analysis of 43 RCTs in orthopedic surgery showed that TXA and aprotinin significantly reduced the need for blood transfusions with an odds ratio of 0.17 for TXA, and 0.43 for aprotinin.7
- Another study looked at TXA use in patients undergoing total joint arthroplasty with a history of coronary artery disease (CAD) and concluded that concerns for myocardial infarction in patients with history of CAD or cardiac stents are unfounded3:
- There were no postoperative myocardial infarctions in patients with CAD or stents.
- Cardiac stents and/or CAD do not preclude the use of antifibrinolytics.
Liver surgery
- Hyperfibrinolysis is a major contributor to coagulation abnormalities during hepatectomy and liver transplantation.1 Liver disease can reduce the clearance of tPA, thus inducing a state of hyperfibrinolysis. During the anhepatic phase of liver transplantation, tPA concentration may be increased.2 However, there is concern regarding the prothrombotic effects of antifibrinolytic agents.
- TXA has been shown to reduce bleeding in liver surgery and is the agent of choice for hyperfibrinolysis.1
- Instead of routine prophylaxis, goal-directed therapy using point-of-care testing to assess for fibrinolysis has been suggested, but this approach has not been shown to be associated with superior outcomes.1,2
Obstetrics
- Antifibrinolytics have been studied for use in postpartum hemorrhage (PPH) in both vaginal and cesarean births.
- The World Maternal Antifibrinolytic (WOMAN) trial was an RCT that evaluated the early administration of TXA (1g IV bolus, repeated within 24h if necessary) for PPH following a vaginal delivery or cesarean delivery in more than 20,000 women from 193 hospitals in 21 countries.8 It showed a reduction in death due to bleeding in patients receiving TXA, although the all-cause mortality and hysterectomy rates were not different between the groups. Deaths from pulmonary embolism, organ failure, sepsis, eclampsia, and other causes did not differ significantly between the groups. No increase in VTE events is noteworthy in the obstetric population, given the baseline hypercoagulable state.
- However, TXA prophylaxis does not reduce the risk of PPH among women undergoing vaginal delivery and may not have a clinically meaningful effect after cesarean delivery.4
- Serious neurological injury can occur after accidental intrathecal injection of TXA. Therefore, TXA vials should not be stored in the same location as other anesthesia drug vials.4
Neurosurgery
- In patients with subarachnoid hemorrhage, antifibrinolytics have been shown to reduce rebleeding with no increased risk of cerebral ischemic events or adverse events.1,2 However, it’s use is limited secondary to concerns for cerebral ischemia and infarction.4
- TXA may reduce blood loss in tumor resection surgery without an increase in thrombotic complications.4
- TXA use in intracranial aneurysm and intracerebral hemorrhage is also limited secondary to concerns for ischemia and no net clinical benefit.4
Pediatric surgery
- TXA use is recommended in children undergoing surgeries with high or moderate risk of bleeding such as cardiac surgery, spinal fusion, craniosynostosis, plastic and maxillary surgeries, major abdominal surgery, etc.4
Risks
A longstanding concern with the use of antifibrinolytic agents is the risk of venous thromboembolic events (VTE). However, there is no data proving a detrimental prothrombotic effect for TXA or EACA1. In RCTs involving antifibrinolytics, there were no increases in thrombotic complications compared to controls in major orthopedic, cardiac, trauma (CRASH-2 trial), and liver transplantation surgery.2
TXA has been associated with postoperative seizures in various settings when used in large doses.1-4 Suggested mechanisms for TXA-induced seizures include neuronal excitation by competitive antagonism of inhibitory neurotransmitters gamma-aminobutyric acid and glycine. In a recent meta-analysis of RCTs, the use of TXA was associated with a 4.1-fold increased risk of clinical seizures.2,3 However, in 2017, the pediatric craniofacial collaborative reported no difference in seizure activities in children receiving antifibrinolytics.9 Seizure disorders are not a contraindication to use TXA in children and adolescents.4
Aprotinin was temporarily removed from the U.S. market in 2008 after it was shown to be associated with increased mortality in the Blood Conservation Using Antifibrinolytics in a Randomized Trial (BART) study for high-risk cardiac surgery.10 The suspension has been recently lifted in some countries after data from the BART study was reviewed.1-4
References
- Ortmann E, Besser MW, Klein AA. Antifibrinolytic agents in current anaesthetic practice. Br J Anaesth. 2013; 111(4):549-63. PubMed
- Levy J, Koster A, Quinones Q, et al. Antifibrinolytic therapy and perioperative considerations. Anesthesiology. 2018; 128 (3): 657-70. PubMed
- Koster A, Faraoni, Levy JH. Antifibrinolytic therapy for cardiac surgery: An update. Anesthesiology. 2015;123(1): 214-21. PubMed
- Patel PA, Wyrobek JA, Butwick AJ, et al. Update on applications and limitations of perioperative tranexamic acid. Anesth Analg. 2022;135(3): 460-73. PubMed
- Lier, H, Maegele, M, Shander, A. Tranexamic acid for acute hemorrhage: A narrative review of landmark studies and a critical reappraisal of its use over the last decade. Anesth Analg. 2019; 129(6): 1574-84. PubMed
- Shakur, H., Roberts, I., Bautista, R., et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet (London, England). 2010. 376(9734), 23–32. PubMed
- Zuffrey P, Mequol F, Laporte S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology.2006;105:1034-46. PubMed
- WOMAN Trial Collaborators (2017). Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2017; 389(10084): 2105–2116. PubMed
- Goobie SM, Cladis FP, Glover CD, et al. Safety of antifibrinolytics in cranial vault reconstructive surgery: a report from the pediatric craniofacial collaborative group. Paediatr Anaesth. 2017 Mar;27(3):271-281. PubMed
- Fergusson DA, Hébert PC, Mazer CD, et al. BART Investigators. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008. 29;358(22):2319-31. PubMed
Other References
- Graetz, T, Nuttall, G, Shander, A. Perioperative Blood Management: Strategies to minimize transfusions. In: Post T, ed. UpToDate; 2022. May 22, 2022.
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.