Copy link
Anticoagulants
Last updated: 07/03/2025
Key Points
- Anticoagulant medications reduce the risk of stroke, systemic embolism, and recurrent venous thromboembolism (VTE).
- Selecting and dosing anticoagulants requires consideration of each patient’s unique medical characteristics, including pregnancy status, renal and hepatic function, age, weight, and potential drug interactions.
Introduction
- Anticoagulants are a subcategory of antithrombotic drugs, which are used to prevent and treat thromboembolic events by blocking specific steps in the coagulation cascade.
- Etiologies of thrombus formation can be derived from the triad of venous stasis, endothelial injury, and disorders of hypercoagulability.1
- Anticoagulants aim to prevent thrombus formation and thereby decrease the incidence of diseases such as VTE, heart valve thrombosis, and embolism formation in patients with atrial fibrillation (AF).1-3
- The use of anticoagulants in clinically appropriate settings reduces both morbidity and mortality.
Drug Classes, Mechanisms of Action, and Monitoring of Effects
There are five primary classes of anticoagulants, and in certain cases, some anticoagulants might be favored over others (Figure 1 and Table 1).1,3
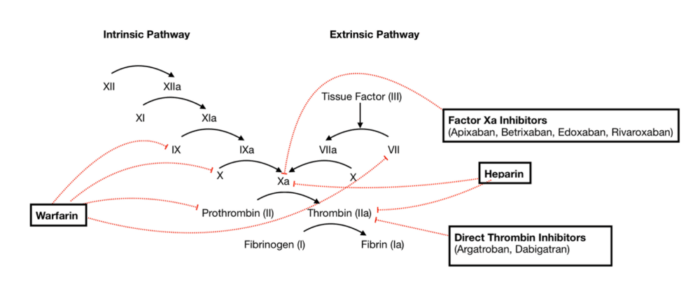
Figure 1. Anticoagulant actions within the clotting cascade. Source: Wikimedia Commons. SteveKong3. CC-BY SA 4.0. Link
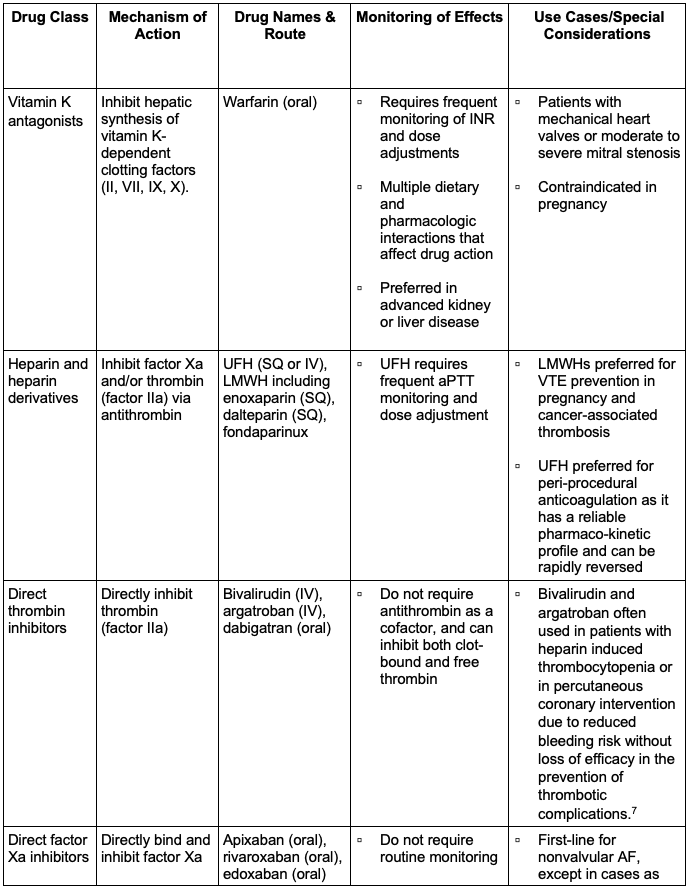

Table 1. Classes of anticoagulants, mechanisms of action, routes of administration, relevant side effect monitoring, and use cases. 1,3,5 Abbreviations: INR, International Normalized Ratio; SQ, subcutaneous; IV, intravenous; LMWH, low molecular weight heparin; DVT, deep vein thrombosis; PE, pulmonary embolism; AF, atrial fibrillation; VTE, venous thromboembolism; aPTT, activated partial thromboplastin time; UFH, unfractionated heparin
Dosages
Vitamin K Antagonists
- In thromboprophylaxis for VTE and AF, warfarin is typically started at a dose of 5 mg daily and titrated to an international normalized ratio (INR) target of 2.0 to 3.0.
- In thromboprophylaxis for patients with mechanical heart valves, an INR of 2.5-3.5 is targeted.1,8
Unfractionated Heparin (UFH)
- For treatment of VTE, an initiating intravenous (IV) bolus of 80 units/kg is followed by a continuous infusion rate of 18 units/kg/hour. This dose should be titrated to an activated partial thromboplastin time of 1.5-2.5 times control.1,8
Low Molecular Weight Heparin (LMWH)
- Enoxaparin dosing for VTE treatment is 1mg/kg every 12 hours or 1.5 mg/kg once daily, administered subcutaneously.
- Enoxaparin dosing for bridging anticoagulation in AF is the same as listed above. However, there are renal dosing adjustments for patients with renal dysfunction. If creatinine clearance is less than 30 mL/min, a patient should receive 1 mg/kg daily.1,8
Direct Oral Anticoagulants (DOACs)
- Apixaban dosing for AF and VTE is 5 mg twice daily; the dose is reduced to 2.5 mg twice daily if the patient has two or more of the following conditions: age greater than 80 years, weight less than 60 kg, or serum creatinine greater than 1.5 mg/dL.
- Rivaroxaban dosing for AF and VTE is 20 mg daily, and the dose is reduced to 15 mg daily if creatinine clearance is 15-50 mL/min.
- Dabigatran dosing for AF and VTE is 150 mg twice daily; the dose is reduced to 75 mg twice daily if the creatinine clearance is 15-30 mL/min.
- Edoxaban dosing for AF and VTE is 60 mg daily; the dose is reduced to 30 mg daily if the patient’s weight is less than 60 kg or their creatinine clearance is 15-50 mL/min.1,8
Side Effects
Vitamin K Antagonists
- Side effects of warfarin include bleeding (particularly with supratherapeutic INR) and skin necrosis. This drug has a very narrow therapeutic window and many drug and dietary interactions. In addition, it requires frequent INR monitoring and dose titration.4
UFH
- UFH side effects include bleeding, osteoporosis with long-term use, hypersensitivity reactions, and heparin-induced thrombocytopenia (HIT), which can result in paradoxical thrombosis.1,3,5,8
LMWH
- LMWH has a similar risk of bleeding when compared to UFH; however, the risk of both HIT and osteoporosis is significantly lower. Careful consideration should be taken in administering LMWH products to patients with renal disease, as the accumulation of these drugs can increase bleeding risk.1,3,5,8
DOACs
- Rivaroxaban, dabigatran, and edoxaban (when given in high doses) have an increased risk of gastrointestinal (GI) bleeding when compared to warfarin. DOACs are preferred in most thromboprophylaxis situations because they have fewer drug-drug interactions and do not require frequent monitoring of other anticoagulants.1,3,5,8
Perioperative Management of Anticoagulants
- Before initiating anticoagulation, patients should be evaluated based on their bleeding risk, thromboembolic event risk, capacity for medication adherence, concurrent medications, renal function, and hepatic function. Generalized risk categories may apply to certain patient populations; however, each patient should be assessed on an individualized basis.3,6
Assessment of Bleeding Risk
- Surgical procedures may be classified by minimal risk, low-to-moderate risk, or high risk.
- Minimal bleeding-risk surgeries (30-day risk of major bleed is approximately 0%) may include dental procedures, cardiac pacemaker insertion, or minimally invasive biopsies.6
- Low-to-moderate risk surgeries (30-day risk of major bleed is 0-2%) include GI procedures such as endoscopy or laparoscopic cholecystectomy.6
- High bleeding-risk surgeries (30-day risk of major bleed is >2%) include major thoracic surgery, kidney biopsy, colon resection, and most major orthopedic surgeries.6
- Additional individualized factors must be considered, such as advanced age, kidney or liver dysfunction, prior stroke, concurrent use of NSAIDs, certain blood dyscrasias, or a history of significant intracranial or GI bleeding.6
An extensive list of surgical procedures, stratified by bleeding risk, can be found in Table 2.
Assessment of Thromboembolic Risk
- The CHA₂DS₂-VASc score can be used to determine the stroke risk for patients with atrial fibrillation, with the recommendation of anticoagulation initiation in patients with a score ≥2 if male or ≥3 if female.
- Patients with a mechanical heart valve are considered to have a high thromboembolic risk and must maintain careful monitoring and management of anticoagulation therapy.
- Patients with recent VTE (within 3 months) on anticoagulation therapy must also be carefully managed due to the risk of recurrent embolic events.1,3,5
Renal Function
- Certain DOACs may require dose adjustment for renal impairment or may be relatively contraindicated depending on the extent of renal dysfunction.
Hepatic Function
- Hepatic dysfunction can alter warfarin metabolism and thus must be evaluated regularly and considered when initiating warfarin therapy.
Bridging
- When a patient is preparing for a surgical procedure, a delicate balance must be struck between minimizing thromboembolic risk and reducing intraoperative and postoperative bleeding risk. Ideally, a patient’s anticoagulation would not be interrupted for a significant length of time before or after the perioperative period.1,3,5
- “Bridging” refers to the concept that a long-acting oral anticoagulant may be replaced temporarily by a short-acting parenteral anticoagulant, typically LMWH or unfractionated heparin.3,5
- Patients on warfarin will be instructed to discontinue their anticoagulation 5-6 days prior to surgery and initiate therapeutic-dose LMWH or UFH when their INR falls below the therapeutic range. They should discontinue LMWH/UFH 4-6 hours prior to surgery and resume postoperatively, with the transition to warfarin occurring when INR is therapeutic once again.3,5
- The American College of Chest Physicians recommends that only patients who are at high risk for thromboembolic events – including those with a CHA₂DS₂-VASc > 7, patients with a mechanical mitral valve, and patients who have had a VTE or stroke within the past 3 months – should undergo bridging of anticoagulation.3,5,6
- Bridging is not recommended for patients who are on DOACs due to the pharmacokinetic profile of this drug class and resulting rapid onset and offset.
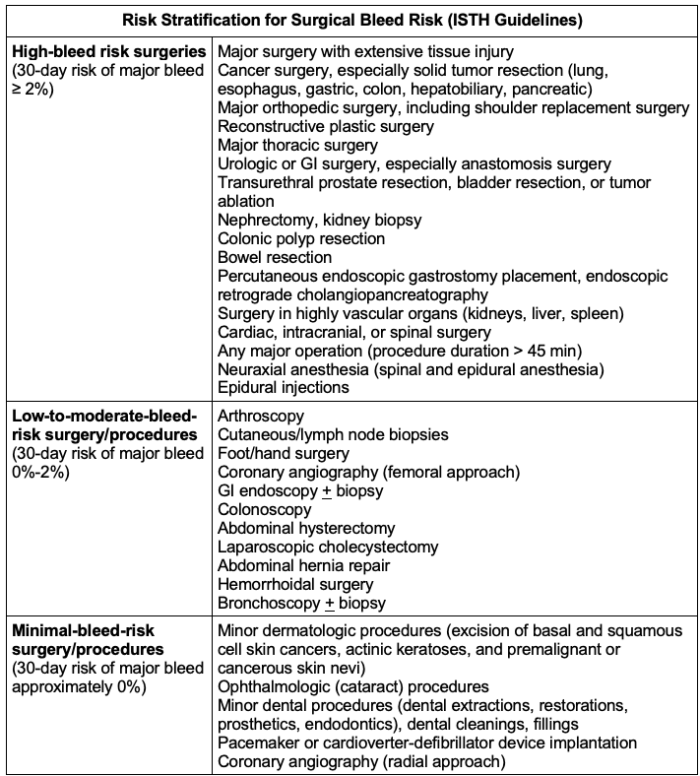
Table 2. Risk stratification for surgical/procedural bleed risk from the International Society on Thrombosis and Haemostasis (ISTH). Adapted from Spyopoulos AC, et al. J Thromb Haemost. 2019; 17(11); 1966-72.6
Table 3 and Figure 2 summarize the guidelines for interrupting anticoagulation before surgical procedures, as well as the timing of resuming anticoagulation postoperatively.
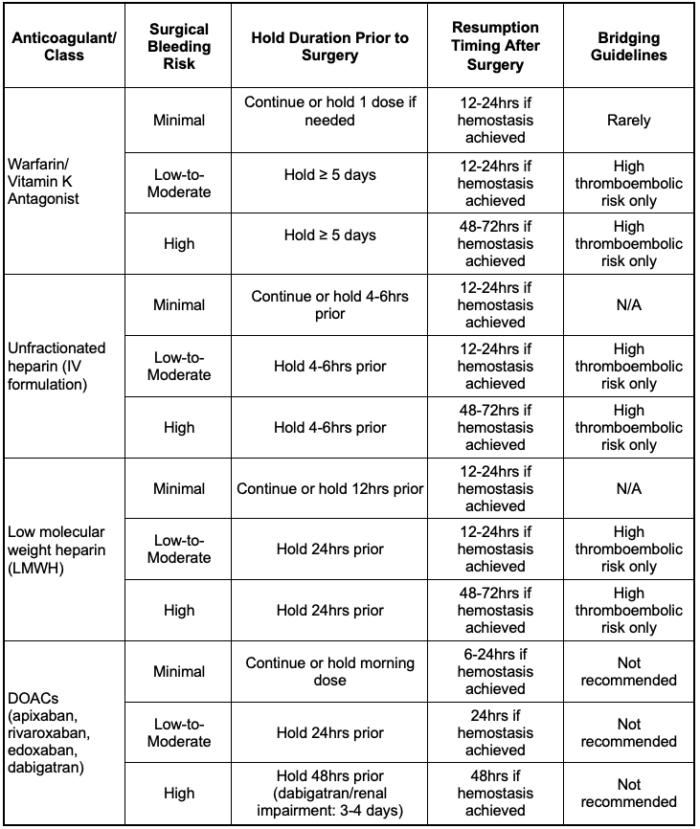
Table 3. Duration of interruption and resumption schedule of anticoagulants by drug class and surgical bleeding risk category.1,3,6 Abbreviation: DOAC, direct oral anticoagulants
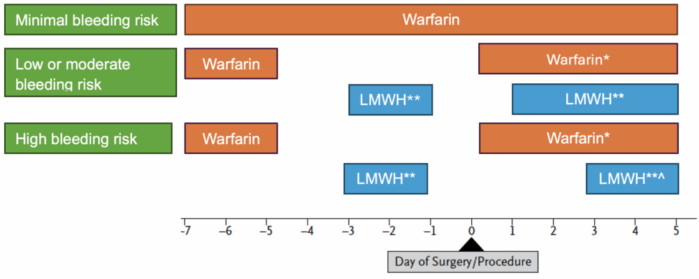
Figure 2. Perioperative management of anticoagulants. Adapted from Douketis JD, et al. Perioperative management of antithrombotic therapy: An American College of Chest Physicians Clinical Practice Guideline. Chest. 2022;162(5):e207-e243.2
* Warfarin can be resumed on the evening of the surgery (day 0) for most patients or on postoperative day 1 at the patient’s usual maintenance dose.
** Bridging therapy is suggested for high thrombotic risk populations with full-dose, subcutaneous LMWH, with the last dose given the morning of the day before the procedure at half the total daily dose.
^ Low-dose LMWH can be used for VTE prophylaxis for the first 24-72 hours postoperatively, with full-dose LMWH resumed 2-3 days postprocedure.
Specialized Anticoagulant Patient Populations and Management
- Certain patient populations require adherence to a separate set of guidelines regarding anticoagulation.
- Please see the OA summary “Management of Pregnant Patients on Anticoagulation” for more details. Link
- Please see the OA summaries “Regional Anesthesia in Patients Rec Antithrombotic or Thrombolytic Therapy”: part 1 (Link) and part 2 (Link)
References
- Douketis JD, Spyropoulos AC. Perioperative management of anticoagulant and antiplatelet therapy. NEJM Evidence. 2023;2(6): EVIDra2200322. PubMed
- Douketis JD, Spyropoulos AC, Murad MH, et al. Perioperative management of antithrombotic therapy: an American College of Chest Physicians clinical practice guideline. Chest. 2022;162(5):e207–e243. PubMed
- Douketis JD, Spyropoulos AC. Perioperative management of patients taking direct oral anticoagulants: a review. JAMA. 2024; 332(10):825-34. PubMed
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2023;148(6):e109-e223. PubMed
- Shah S, Nayfeh T, Hasan B, et al. Perioperative management of Vitamin K antagonists and direct oral anticoagulants. Chest. 2023;163(5):1245–1257. PubMed
- Spyropoulos AC, Brohi K, Caprini J, et al. Scientific and standardization committee communication: Guidance document on the periprocedural management of patients on chronic oral anticoagulant therapy: recommendations for standardized reporting of procedural/surgical bleed risk and patient-specific thromboembolic risk. J Thromb Haemost. 2019: 17(11): 1966-72. PubMed
- Lehman SJ, Chew DP. Bivalirudin in percutaneous coronary intervention. Vasc Health Risk Manag. 2006;2(4):357-63. PubMed
- Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2_suppl):e152S-e184S. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.