Copy link
Anesthesia for Pediatric Liver Transplantation
Last updated: 11/28/2023
Key Points
- Indications for pediatric liver transplantation (LT) differ greatly from adult LT. Pediatric patients may present with underlying metabolic or genetic diseases, which may pose additional challenges in anesthetic management. Please see the OA Summary on adult LT. Link.
- Pediatric patients may have end-stage liver disease (ESLD) and its typical sequelae or may present with normal hepatic function and coagulation status, e.g., genetic, metabolic, or neoplastic indications.
- Key differences exist in the intraoperative management of pediatric LT as compared to adult LT, and each surgical phase has several unique considerations.
- Following transplantation, pediatric LT recipients are at a much higher risk of hepatic artery thrombosis as compared to adults.
Introduction
- The first pediatric LT was performed by Dr. Thomas Starzl and colleagues in 1963 at the University of Colorado.1 Currently, more than 500 pediatric transplants are performed in the United States each year across more than 60 centers.2
- Historically, 60% of all children listed for LT are aged 5 years or younger.
- Pretransplant mortality is highest in patients younger than 1 year and in those of Asian or Black ethnicity.2
- The most common indication for pediatric LT is biliary atresia (BA), followed by genetic or metabolic disease and toxic or infectious causes (Figure 1).1
- Careful perioperative planning and communication with a multidisciplinary team are vital to ensure the best care for pediatric LT recipients.1
- The Pediatric model for End-stage Liver Disease (PELD Cr) is a numeric scale used to quantify disease severity for transplant candidates younger than 12 years. The Model for End-Stage Liver Disease (MELD 3.0) is used for children aged 12 and older and adults. Demographic and clinical components of both scales are compared in Table 1. PELD Cr and MELD 3.0 calculators can be found here.3
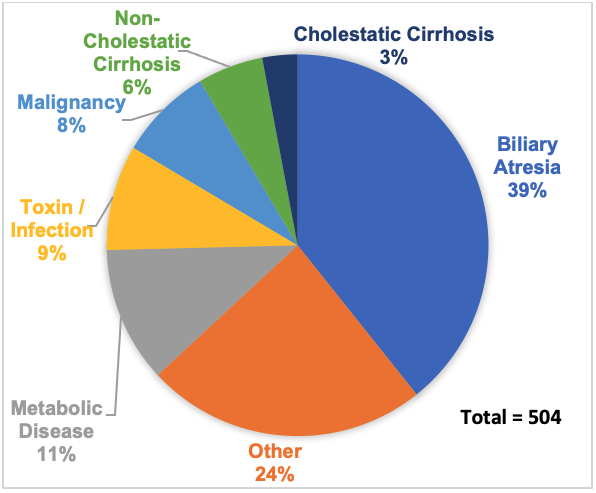
Figure 1. Indications for Pediatric Liver Transplant, 2021. Adapted form from Organ Procurement and Transplantation Network (OPTN). Health Resources and Services Administration, US Department of Health & Human Services. Available at: Link.
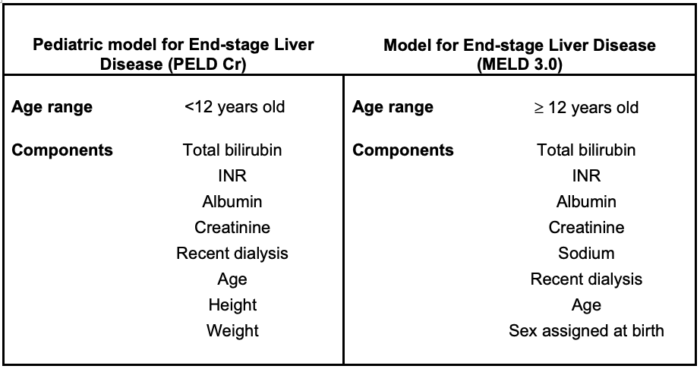
Table 1. Characteristics of scoring systems in pediatric liver transplantation. Adapted from PELD and MELD calculators found at Liver policy: medical urgency. Unos.org.
Preoperative Considerations
- Pediatric LT candidates have comorbidities that may or may not be related to their transplant need, e.g., Alagille’s syndrome, congenital cardiac disease, or inborn errors of metabolism.4
- A multidisciplinary team, including psychology and social work, should meet to discuss surgical and postoperative risks.
- There are several absolute contraindications to pediatric LT (Table 2).2
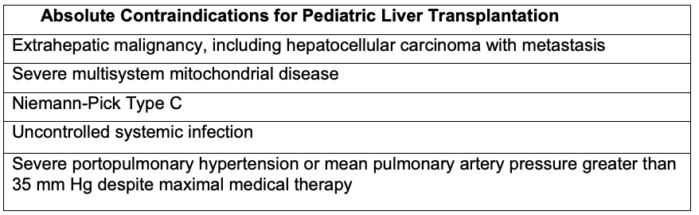
Table 2. Absolute contraindications for pediatric liver transplantation. Adapted from Wasson NR, et al. Anesthetic management of pediatric liver and kidney transplantation. Anesthesiology Clin. 2017: 421-38. PubMed.
- Assessment of the patient’s and guardians’ compliance with treatment (including clinic visits, vaccinations, and medications) is important.
- Pediatric LT patients may or may not present with significant sequelae of ESLD. In the case of genetic or metabolic indications for LT, patients may present with normal hepatic function and/or coagulation status.
- Candidates for LT are at risk for several laboratory abnormalities, including hyperkalemia, hyponatremia, hypo- or hyperglycemia, anemia, thrombocytopenia, and/or coagulopathy.4
- If patients have significant electrolyte derangements or renal dysfunction preoperatively, the ability to perform intraoperative dialysis should be arranged.
- Blood product availability should be arranged prior to the procedure. Patients with previous abdominal procedures, e.g., Kasai procedure, are at an increased risk of significant blood loss during the dissection phase.
- Patients may also have had multiple peripheral and central venous lines. This should be considered when planning for potential hemorrhage and vascular access.
- Anesthesia preoperative evaluation should include, at minimum, the reason for transplant, current hepatic and renal function (including coagulation status and sequelae of portal hypertension), cardiopulmonary comorbidities, prior abdominal surgeries and venous access, and special considerations for specific underlying diseases (Table 3).
- Pediatric LT patients with metabolic diseases may have specific glucose management requirements during the procedure.
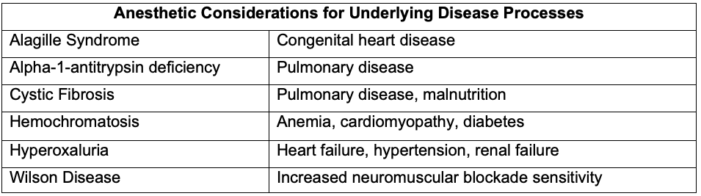
Table 3. Anesthetic considerations for underlying disease processes. Adapted from Wasson NR, et al. Anesthetic management of pediatric liver and kidney transplantation. Anesthesiology Clin. 2017: 421-38. PubMed.
Intraoperative Considerations
The surgical phases of LT, as well as common anesthetic considerations for each phase, are discussed in detail elsewhere (see the OA Summary on adult LT Link). The focus of this section will be differences from adult LT.
Induction
- Preoperative anxiety is common in pediatric patients, and intravenous (IV) or oral midazolam is usually well tolerated. Anxiolytic treatment is usually avoided in patients with encephalopathy. Caution should be used for intramuscular anxiolysis in patients with coagulopathy.4
- Infants and younger children are at a higher risk for hypothermia during the induction/lines period, which may negatively impact preexisting coagulopathy; care should be taken to avoid this problem with ambient warmers or an underbody forced-air warmer.
- In patients who are not appropriately fasted or have significant gastroparesis and/or ascites, full stomach precautions should be considered and IV access should be obtained prior to induction to facilitate rapid sequence induction. However, in patients without these concerns, mask induction with sevoflurane is acceptable.
- Propofol, etomidate, or ketamine may all be used for IV induction, depending on comorbidities. Fentanyl is commonly used for analgesia during induction and maintenance, as its half-life is unchanged in liver disease.4
Access and Monitoring
- At least 2 large-bore peripheral IVs should be placed in the upper extremities as the IVC may be clamped during the anhepatic phase. Ultrasonography or other vein-finding modalities should be used as needed. Rapid infusion catheters (RICs) should be considered in older children.
- In children, central blood administration carries a higher risk of hyperkalemic arrest, and peripheral administration is commonly preferred.5
- In addition to standard American Society of Anesthesiologists monitors, radial or ulnar arterial catheterization is usually performed to facilitate close hemodynamic monitoring and allows the drawing of frequent arterial blood gas samples. Femoral catheterization may be performed but has been associated with limb ischemia in children, and the potential for aortic cross-clamping would render the line useless.1
- Central access should be obtained for central venous pressure (CVP) monitoring and vasoactive infusions. At some institutions, interventional radiology assists with line placement beforehand.1 Caliber and length of the line is dependent on patient size and weight. Internal jugular or subclavian veins are preferable locations; caution should be used in patients with coagulopathy.
- Transesophageal echocardiography is frequently used for adult LT but is not routinely used for pediatric LT. It may have use in patients with preexisting cardiac disease or to assist in diagnosing unstable hemodynamics not otherwise explained.1
Bleeding Risk
- Less than 30% of pediatric LT patients require allogenic packed red blood cells (pRBC) transfusion.6
- Pediatric LT patients with a history of abdominal surgery (e.g., Kasai procedure or previous LT) may have significant bleeding during the dissection phase due to adhesions.
- Thromboelastogram (TEG) and rotational thromboelastometry (ROTEM) are commonly used to monitor coagulation status, and evidence supports the use of these tests to decrease transfusion.1 Preoperative coagulation studies may be especially helpful in guiding transfusion in patients with impaired synthetic function.
Anhepatic Phase
- The hemodynamic effects of inferior vena cava (IVC) cross-clamping may be more pronounced in children who lack significant portal hypertension and collaterals, i.e., those undergoing LT for a metabolic disorder, toxins, or infectious hepatitis.7
- Hypotension is preferentially treated with vasopressors during this phase to avoid the risk of volume overload and graft congestion following cross-clamp release.
- Hypothermia is especially common during graft introduction with core temperature decreasing 1-2°C.6 This can have further deleterious effects on the coagulation status.
- Electrolyte abnormalities are common and include, but are not limited to, acidosis from cessation of lactate metabolism, hyperkalemia from acidosis and transfusion, and hypoglycemia from lack of gluconeogenesis and insulin metabolism. Close monitoring with point-of-care (POC) testing is key to recognizing brisk metabolic changes.
- Though hyperkalemia is slightly less common in the pediatric LT population,7 it is recommended to keep potassium levels below 4 mEq/L in preparation for reperfusion.
- Hypocalcemia may result from the inability to metabolize citrate, causing a decrease in vascular tone and heart contractility.7 This effect is especially pronounced in infants.
- Size discordance between the donor liver and recipient in pediatric LT can result in more severe hypotension from reperfusion as blood fills the large organ and decreases intravascular volume.7
Neohepatic Phase
- Maintaining euvolemia in pediatric patients during this phase can be challenging, with fluid overload risking graft congestion and intestinal edema. Conversely, hypovolemia could result in graft hypoperfusion.
- Close discussion with the surgical team regarding volume status is paramount, as the surgeons can directly observe the IVC flow to provide feedback.7
- Studies on fluid responsiveness in pediatrics have assessed several methods, including variables derived from pressure wave measurements (such as pulse pressure variation or stroke volume variation); however, only respiratory variation of aortic blood flow peak velocity has consistently shown a predictive ability in this population.8
- Compared with adults, children undergoing LT are more hypercoagulable after LT, and thus transfusion thresholds and coagulopathy management may vary.
- Hepatic artery thrombosis (HAT) is as much as four times more frequent than in adult transplant patients.1
- Discussion with the surgical team regarding the patient’s coagulation status prior to attempting to correct coagulopathy is crucial to balance the patient’s risk of bleeding with the risk of thrombosis.
- Unlike adults, pediatric LT patients may receive either a Roux hepaticojejunostomy or a traditional choledochocholedochostomy (duct-to-duct) anastomosis.
- Size discordance can contribute to increased abdominal pressure at the time of abdominal wall closure, decreasing graft perfusion and potentially leading to abdominal compartment syndrome.7 Observation of respiratory pressures during closure can increase the suspicion of this occurrence.
Postoperative Considerations
- Patients should be monitored postoperatively in a pediatric intensive care unit for signs of bleeding, graft function, thrombosis, and respiratory and/or hemodynamic complications.
- Early extubation in the operating room is safe for most pediatric LT patients.9
- If mechanical ventilation is needed, limiting positive end-expiratory pressure and allowing spontaneous or supported ventilation strategies are encouraged to limit liver parenchymal congestion.
- There is no evidence that any particular sedation medication is most favorable postoperatively; however, limiting sedation and paralysis to enable spontaneous ventilation is encouraged (see above).
- Dexmedetomidine infusion may contribute to significant sedation and/or bradycardia in the setting of delayed graft function, given its dependence on hepatic function for clearance.10
References
- Wasson NR, Deer JD, Suresh S. Anesthetic management of pediatric liver and kidney transplantation. Anesthesiology Clin. 2017: 421-38. PubMed
- Kwong, AJ, Ebel, NH, Kim, WR, et al. OPTN/SRTR 2020 Annual Data Report: Liver. Am J Transplant. 2022; 22 (Suppl 2): 204 -309. PubMed
- Liver policy: medical urgency. Unos.org. Accessed November 14, 2023. Link
- Fuhr PG, Wilder MS, Bielsky AR. Anesthetic considerations for the child undergoing transplantation. In SP Dunn and Horslen S (Eds.), Solid Organ Transplant in Infants and Children. Heidelberg, Germany, Springer International Publishing.
- Bhananker SM, Ramamoorthy C, Geiduschek JM, et al. Anesthesia-related cardiac arrest in children: update from the Pediatric Perioperative Cardiac Arrest Registry. Anesth Analg 2007; 105:344–50. PubMed
- Tran LT, Mazariegos GV, Damian D, et al. Red blood cell transfusion in pediatric orthotopic liver transplantation: What a difference a few decades make. Anesth Analg. 2019; 129(4): 1087-92. PubMed
- Ballard HA, Jones E, Malavazzi Clemente MM, et al. Error traps in anesthesia for pediatric liver transplantation. Paediatr Anaesth. 2022;32(12):1285-91. PubMed
- Lee JH, Kim EH, Jang YE, et al. Fluid responsiveness in the pediatric population. Korean J Anesthesiol. 2019;72(5):429-40. PubMed
- Yoeli D, Nguyen T, Wilder MS, et al. Immediate extubation following pediatric liver transplantation. Pediatric Transplant. 2022;26(8):e14352. PubMed
- Banc-Husu AM, Badke CM, Sanchez-Pinto LN, et al. Dexmedetomidine leading to profound bradycardia in a pediatric liver transplant recipient. Pediatr Transplant. 2021;25(5):e13895. PubMed
Copyright Information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.